A kind of preparation method of nadroparin calcium
A technology of nadroparin calcium and heparin sodium, which is applied in the field of pharmaceutical synthesis, can solve the problems of cumbersome process operation, high proportion of drying weight loss, and low anti-Xa/anti-IIa ratio, so as to reduce process steps, improve preparation efficiency, and reduce drying The effect of weight loss ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
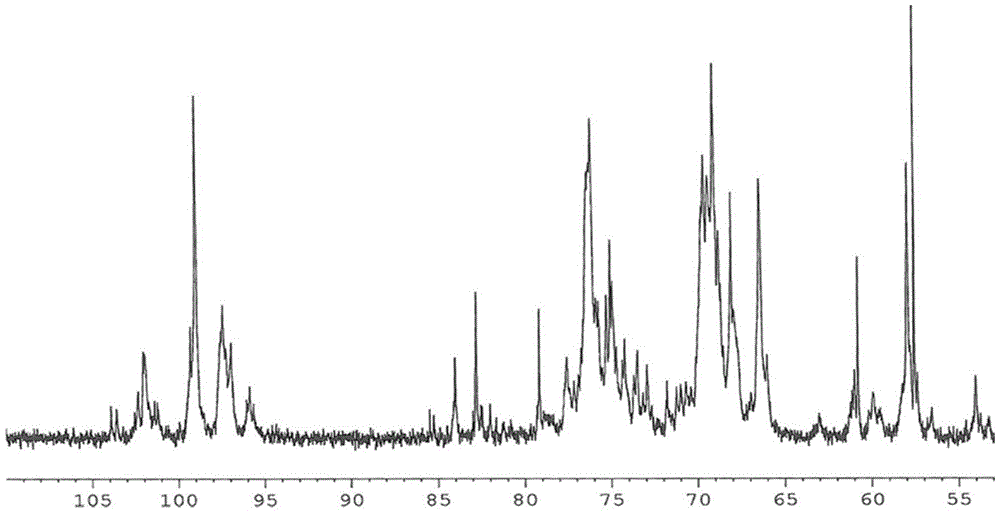
Image
Examples
preparation example Construction
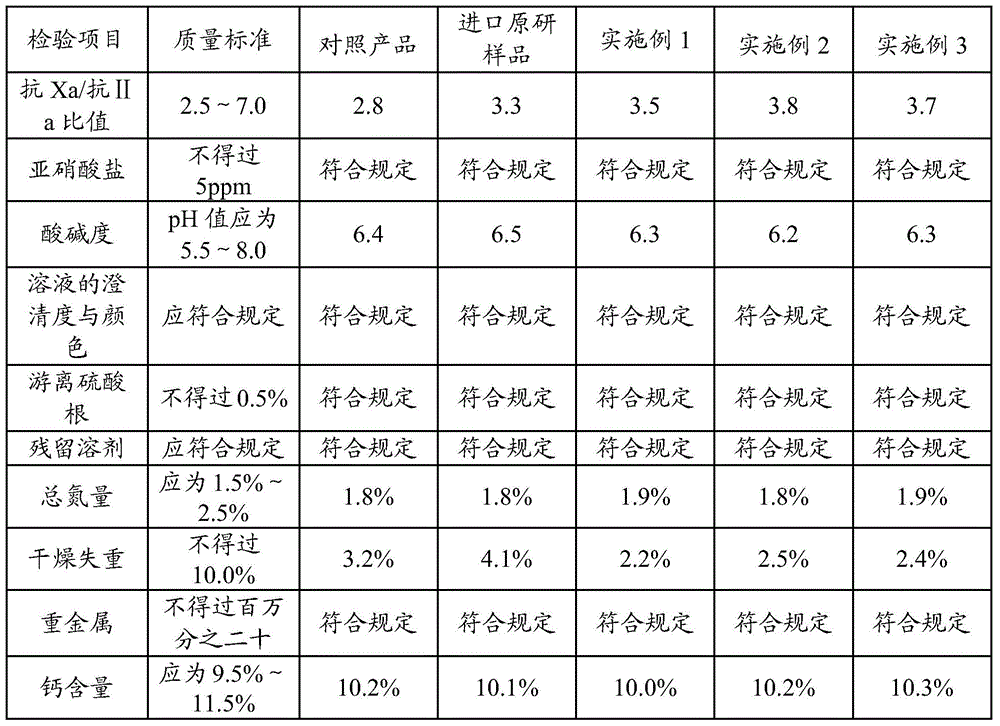
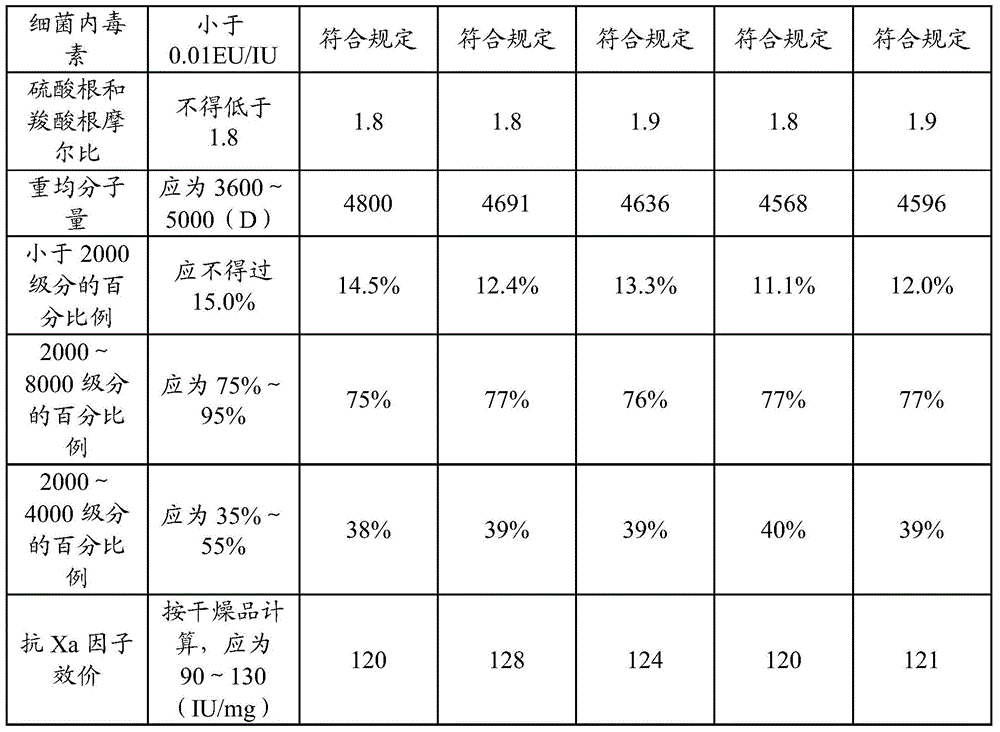
[0029] The invention discloses a preparation method of nadroparin calcium, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The preparation method of the present invention has been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the methods and applications described herein without departing from the content, spirit and scope of the present invention to achieve and Apply the technology of the present invention.
[0030] A preparation method of nadroparin calcium provided by the present invention will be further described below.
Embodiment 1
[0031] Embodiment 1: preparation nadroparin calcium
[0032] Take 4 kg of refined heparin sodium, add 100-200 L of water to dissolve, adjust the pH of the solution to 4 with glacial acetic acid, then add 114 g of sodium nitrite to the solution, and stir for 5 hours at a temperature of 25 ° C to obtain a heparin sodium degradation solution. The prepared heparin sodium degradation solution was adjusted to pH 8 with sodium hydroxide, added 100 g of sodium borohydride (2.5% heparin sodium mass) for reduction for 10 h, and adjusted to pH 7 with glacial acetic acid to obtain a reduced solution.
[0033] Filter the reducing solution with a 0.22 μm filter membrane, add 0.5mol / L calcium chloride solution to the filtrate, stir evenly, and perform ultrafiltration with a 1000D ultrafiltration membrane to complete sodium-calcium replacement. Then add water for injection for ultrafiltration, repeat 2-6 times.
[0034] Add 95% ethanol to the ultrafiltration filtrate for precipitation, colle...
Embodiment 2
[0037] Embodiment 2: preparation nadroparin calcium
[0038] Take 4 kg of refined heparin sodium, add 100-200 L of water to dissolve, adjust the pH of the solution to 6 with glacial acetic acid, then add 132 g of sodium nitrite to the solution, and stir for 6 hours at a temperature of 20 ° C to obtain heparin sodium degradation solution. The prepared heparin sodium degradation solution was adjusted to pH 12 with sodium hydroxide, added 20 g of sodium borohydride (0.5% heparin sodium mass) for reduction for 16 hours, and then adjusted to pH 5 with glacial acetic acid to obtain a reduced solution.
[0039] Filter the reducing solution with a 0.22 μm filter membrane, add 0.1mol / L calcium chloride solution to the filtrate, stir evenly, and perform ultrafiltration with a 5000D ultrafiltration membrane to complete sodium-calcium replacement. Then add water for injection for ultrafiltration, repeat 2-6 times.
[0040] Add 95% ethanol to the ultrafiltration filtrate for precipitation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com