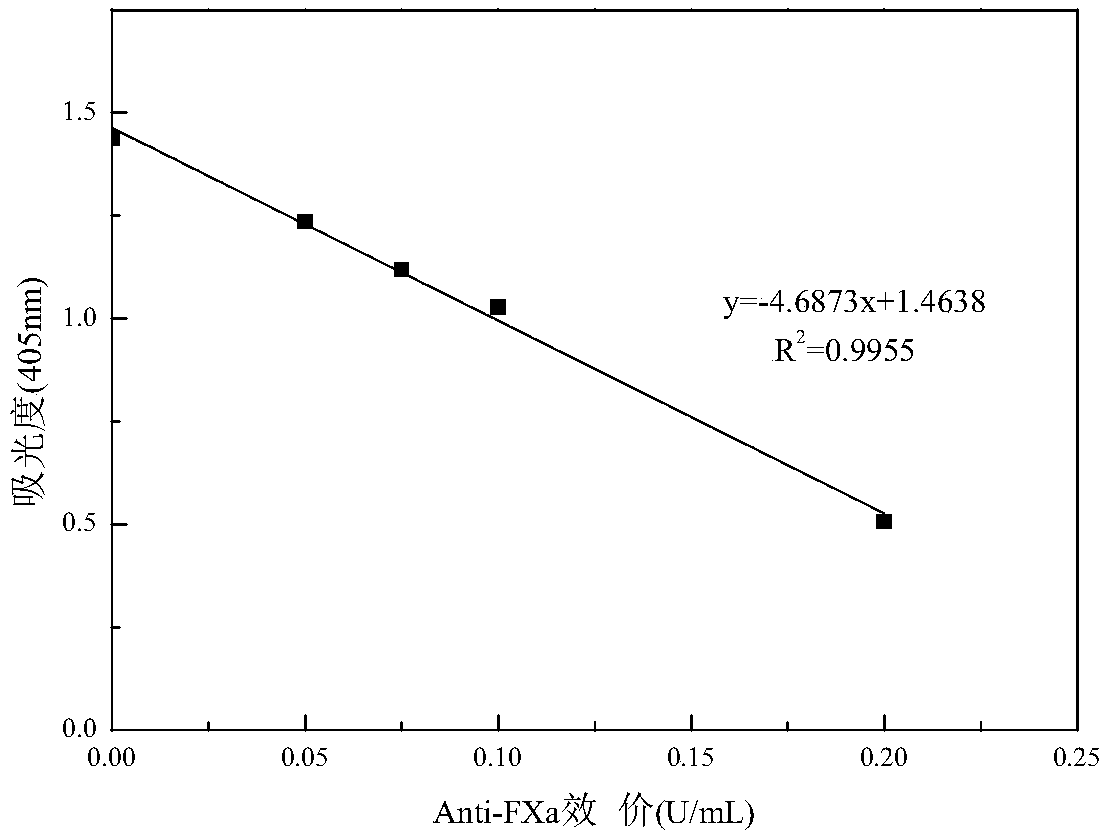
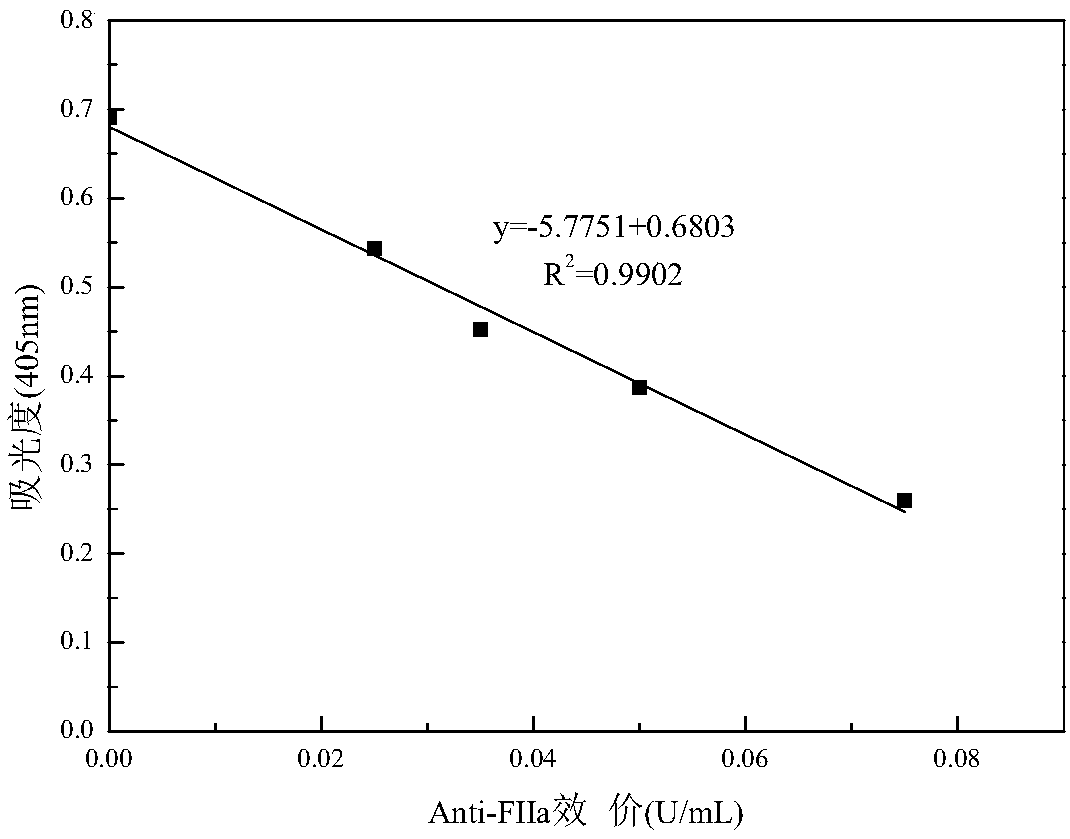
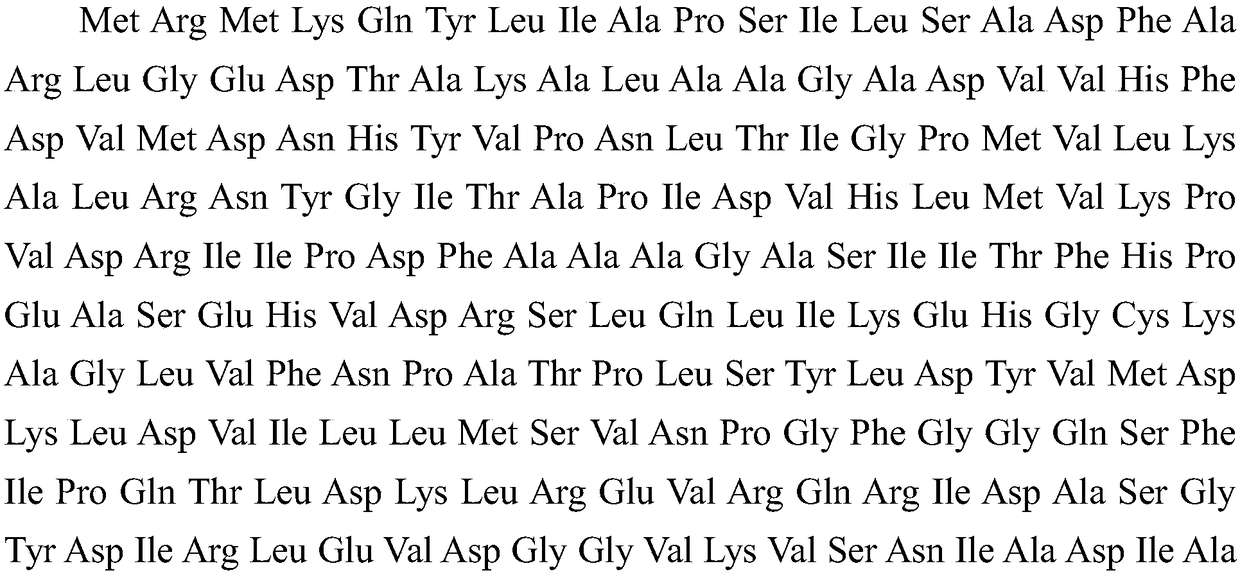
Escherichia coli producing heparinase, construction method and application thereof
A technology of Escherichia coli and its construction method, applied in the field of Escherichia coli producing heparanase and its construction, can solve the lack of systematic research on the production technology of heparinase and heparin drugs produced by recombinant bacteria And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] In the present invention, the preparation method of the heparinase-producing Escherichia coli seed liquid preferably includes: inoculating the heparinase-producing Escherichia coli in a seed medium for shaking culture to OD 600 The value is 1 to 2. In the present invention, the conditions of the shaking culture include: the temperature of the shaking culture is preferably 35 to 37° C., and the rotation speed of the shaking culture is preferably 150 to 250 rpm, more preferably 180 to 220 rpm, and most preferably 200 rpm.
[0081] In the present invention, the seed culture medium preferably uses water as the solvent, and preferably includes: 10-15g tryptone, 5-10g yeast extract and 10-15g sodium chloride per liter; more preferably 11-14g tryptone , 6-9g yeast extract and 11-14g sodium chloride; most preferably including 12-13g tryptone, 7-8g yeast extract and 12-13g sodium chloride. In the present invention, the pH value of the seed culture medium is preferably 7.0-7.2.
[00...
Embodiment 1
[0093] Acquisition and preservation of engineered bacteria
[0094] 1. Construction of engineering bacteria
[0095] 1. Using Raoultella sp.NX-TZ-3-15 with the deposit number of CGMCC No. 13723, the bacterial genome DNA rapid extraction kit was used to extract the whole genome DNA of the bacteria.
[0096] 2. According to the sequence of the heparinase produced by the bacteria and the related gene sequence of the plasmid pBENT, according to the principle of seamless cloning, PCR primers are designed in the multiple cloning site region of the plasmid, including the upstream primer SEQ ID NO of the target gene. 1 and the downstream primer SEQ ID NO. 2 and the upstream primer SEQ ID NO. 3 and downstream primer SEQ ID NO. 4 of the plasmid.
[0097] 3. Use the PCR primers designed in step 2 for PCR amplification:
[0098] (1) PCR amplification of target gene: template target gene DNA 100ng / μL 1μL, primer SEQ ID NO.1 is 10μM 0.5μL, SEQ ID NO.2 is 10μM 0.5μL, PCR Premix (2x) enzyme 25μL, ddH ...
Embodiment 2
[0107] Application of engineering bacteria to produce heparinase
[0108] 1. Preparation of culture medium
[0109] Seed culture medium (pH7.0): Take 10g tryptone, 5g yeast extract and 10g sodium chloride, dissolve in water and dilute to 1L; sterilize at 121°C for 30min.
[0110] Fermentation medium (pH7.0): Take 10g tryptone, 5g yeast extract, 10g sodium chloride, dissolve in water and dilute to 1L; sterilize at 121°C for 30min.
[0111] 2. Application of engineered bacteria to produce heparinase
[0112] 1. Inoculate Escherichia coli BLR(DE3)-pBENT-H1 to the seed medium, and shake culture (200r / min) at 35℃ to OD 600nm =1, which is the seed liquid.
[0113] 2. Use a pipette to inoculate the seed solution of step 1 into 10 mL fermentation medium (50 mL shake flasks can be used) at an inoculum amount of 10% (v / v) to obtain OD 600nm =0.2 fermentation initial system; the fermentation process is as follows: first 37°C shaking culture (200r / min) for 2 hours, then add IPTG to the final con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com