Calcium phosphate-lipid nano-drug co-delivery system consisting of low molecular weight heparin and prodrug of natural drug
A low-molecular-weight heparin and natural drug technology, applied in the field of pharmaceutical preparations, can solve the problems of chemotherapy drugs cannot effectively enter tumor tissue, chemotherapy drugs have systemic toxicity, and cannot be targeted, and can reduce the formation of lung metastases, inhibit the The effect of tumor angiogenesis and prolongation of survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
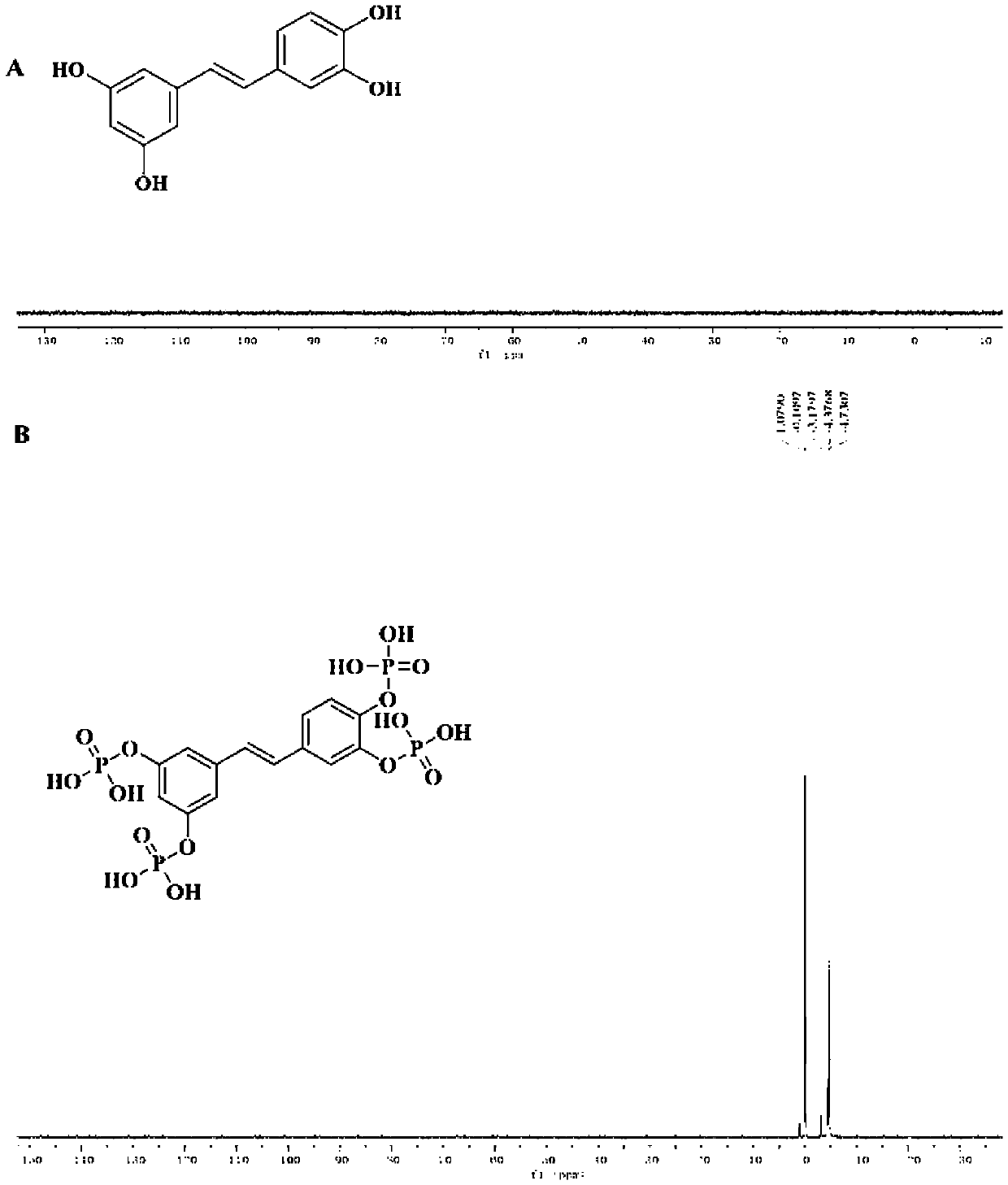
[0041] Example 1: Synthesis and characterization of PIC-POOH
[0042]PIC-POOH was synthesized using a two-step chemistry. The first step, weigh 200mg of PIC solid powder, 780mg of 4-dimethylaminopyridine (DMAP), and take 0.89ml of triethylamine in anhydrous 20ml of tetrahydrofuran with a syringe, add 3ml of dichlorophosphoric acid dropwise after stirring Ethyl ester, nitrogen protection after adding, heated to 70 ℃ reflux, terminated the reaction after 24h, and then purified by silica gel column chromatography to remove reaction impurities and by-products to obtain the intermediate product phosphorylated piceatanol (PIC-POOET ); second step, weigh 50mg of PIC-POOET, dissolve in 5ml of dichloromethane, add 0.33ml of bromotrimethylsilane dropwise after stirring, stir at room temperature for 4h, and remove dichloromethane by rotary evaporation, add methanol and stir for 30min Finally, the reaction is terminated, and then purified by a semi-preparative chromatographic column to r...
Embodiment 2
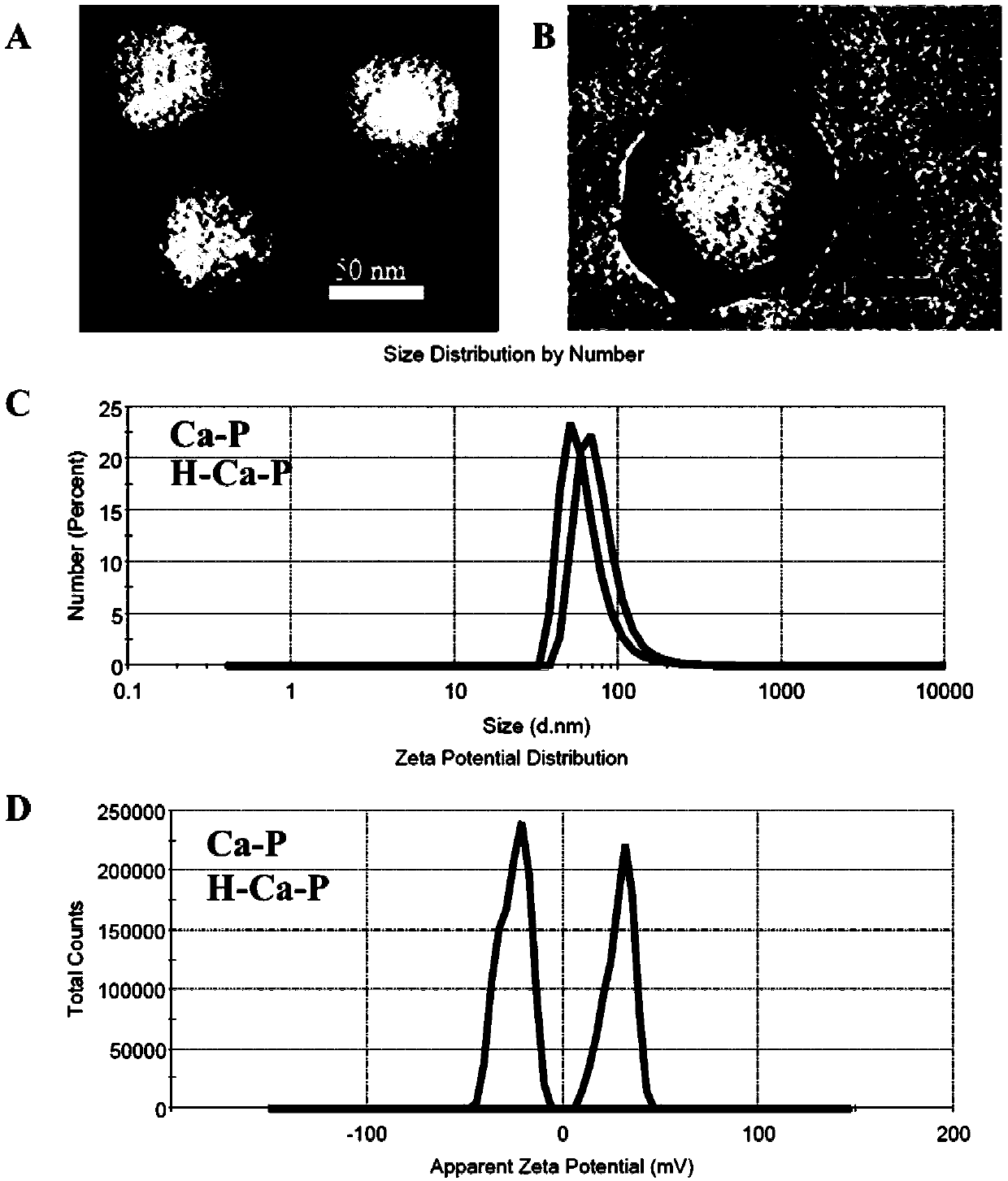
[0044] Example 2: Preparation of calcium phosphate-lipid nanoparticles encapsulated by low molecular weight heparin
[0045] Calcium phosphate nano-cores were prepared by reverse microemulsion method in which PIC-POOH solution and calcium chloride solution were combined in oil phase. Calcium phosphate-lipid nanoparticles were prepared by film hydration method containing calcium phosphate nano-cores: Weigh 16.8mg of DSPE-PEG2000, 8.6mg of cationic lipid material DOTAP and 4.6mg of cholesterol, add 2ml of chloroform to dissolve them Dissolve, add 0.7ml of calcium phosphate nano-precipitate dispersed in chloroform, mix well and add to a 500ml round bottom flask, remove chloroform by rotary evaporation, add 6ml of distilled water to hydrate the lipids attached to the inner wall of the bottle, ice bath Ultrasound for 2.4 minutes under the condition of ultrasonic power 240W, interval 2S, that is to make Ca-P, take it out for use;
[0046] The LMWH was wrapped on the outside of Ca-P...
Embodiment 3
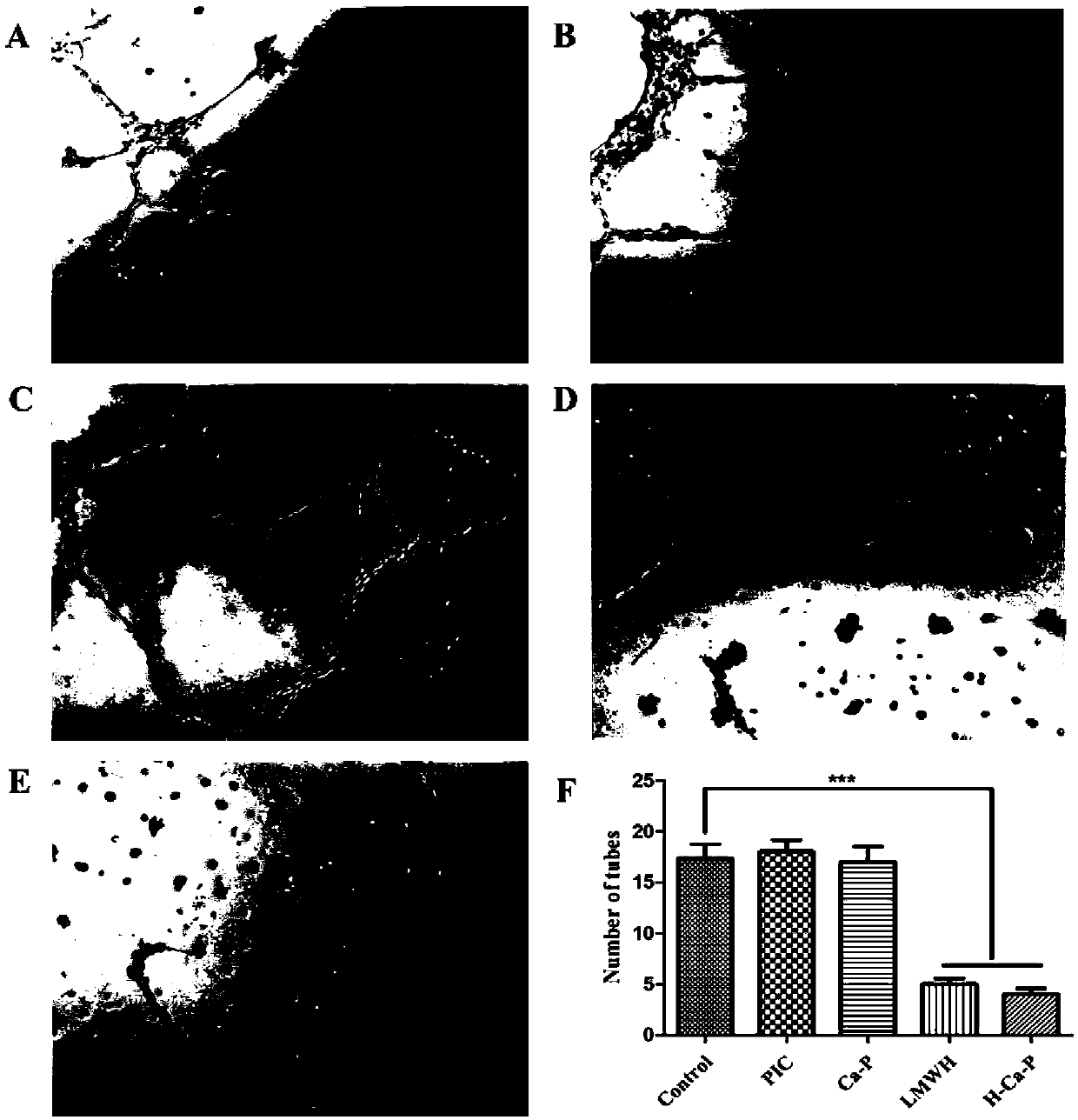
[0048] Example 3: Evaluation of Drug Co-delivery System for Inhibiting Tumor Angiogenesis
[0049] Tubule formation experiments were used to simulate tumor angiogenesis in vivo. After the Matrigel was thawed at 4°C, 50uL was added to a pre-cooled 96-well plate in an ice bath, transferred to 37°C and incubated for 30min until the Matrigel was polymerized. Add 1×10 per well 4 1 HUVEC cells were centrifuged to remove the supernatant, and then resuspended with DMEM solution containing PIC, LMWH, Ca-P, and H-Ca-P (the concentration of PIC and PIC-POOH was 5 μmol / L, and the concentration of LMWH was 3.5 μmol / L). Suspended and inoculated into 96-well plates precoated with Matrigel. The control group used DMEM solution without drugs. After co-incubating for 12 hours, observe and take pictures under a phase-contrast microscope, and use Image J 1.46version software to quantify the number of lumens of tubules formed in each group in the field of view;
[0050] The results showed that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com