NO-loaded docetaxel nano-drug as well as preparation method and application thereof
A technology of docetaxel and nano-medicine, which is applied in the field of NO-loaded docetaxel nano-medicine and its preparation, which can solve the problems of poor water solubility, insufficient targeting, and easy drug resistance of docetaxel. Improved kinetic properties, high drug loading, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Synthesis of isosorbide mononitrate docetaxel conjugate (ISMN-DTX), the process is as follows:
[0030] At room temperature, isosorbide mononitrate and succinic anhydride were placed in an eggplant-shaped flask, dissolved in dichloromethane, and then 4-dimethylaminopyridine (DMAP) was slowly added, and the reaction was stirred at room temperature. After the completion of the reaction monitored by TLC, 20 mL of water was added to the reaction solution, the mixture was extracted with ethyl acetate (20 mL×3), the organic layers were combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure , and purified by silica gel column chromatography to obtain the intermediate.
[0031] At room temperature, put docetaxel and the intermediate in an eggplant-shaped flask, add 10 mL of dry N,N-dimethylformamide, and slowly add 1-ethyl-3-(3-dimethylpropylamine) under an ice bath Carbodii...
Embodiment 2
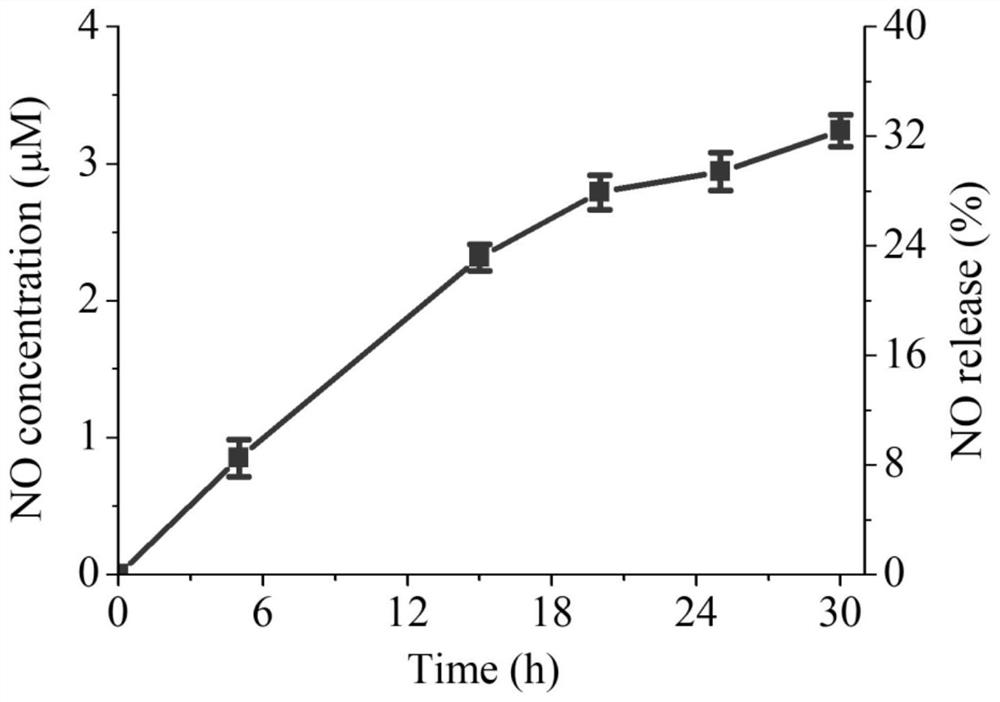
[0033] Example 2: Preparation of NO-loaded Docetaxel Nanomedicine and Determination of Particle Size
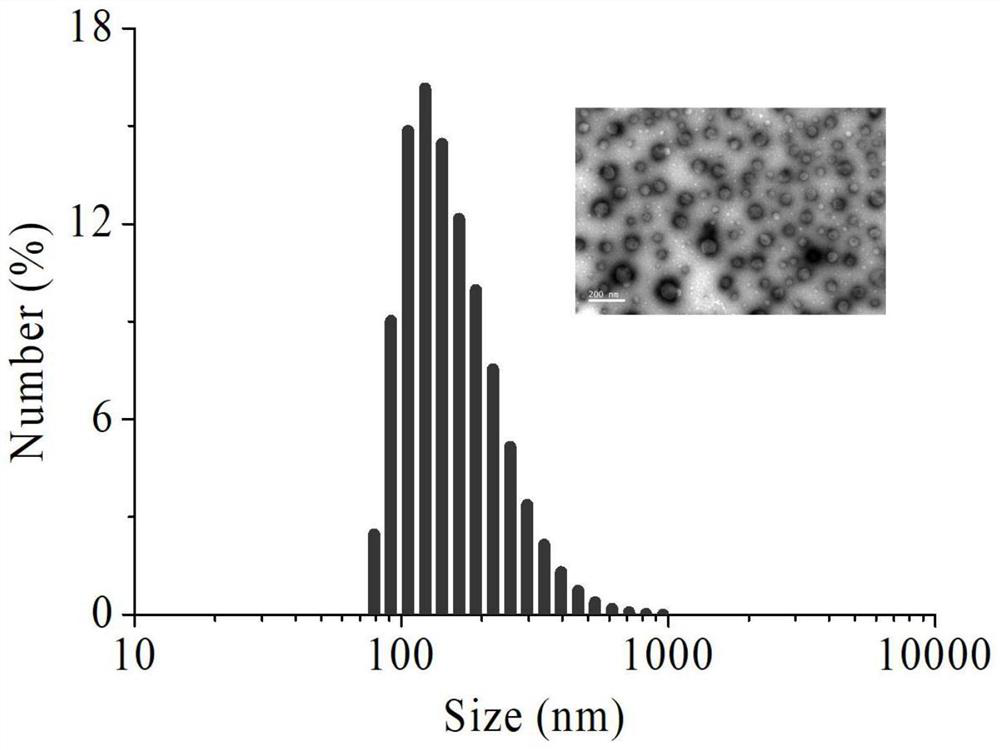
[0034] The NO-loaded docetaxel nanomedicines were prepared by solvent exchange method. First prepare a 4 mg / mL solution of ISMN-DTX in ethanol. 100 μL of the ISMN-DTX solution was then mixed with 40 μL of a 5% solution of vitamin E polyethylene glycol succinate (TPGS) in ethanol. Deionized water was added dropwise under ultrasonic conditions, and the final concentration was controlled to be 0.4 mg / mL. Thereafter, dialysis was performed against deionized water (Spectra / Pore, MWCO 1000). The particle size and appearance of the NO-loaded docetaxel nanomedicine were observed by dynamic light scattering and transmission electron microscopy. The results showed that the prepared nanomedicine could be formed into monodisperse nanoparticles with a hydrated particle size of 164.5 nm and a particle size distribution index of 0.195. Electron microscopy results show that the nanomedici...
Embodiment 3
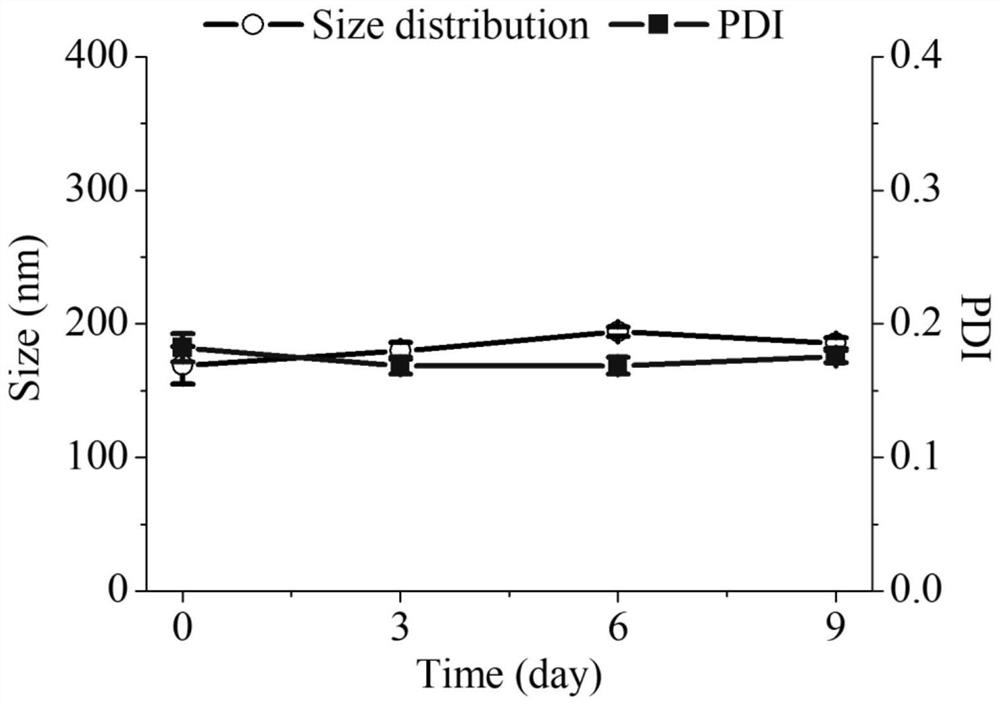
[0035] Example 3: Evaluation of the stability of NO-loaded docetaxel nanomedicines
[0036] In order to study the storage stability of NO-loaded docetaxel nanomedicines, the size of the prepared nanomedicines was measured on day 0, day 3, day 6 and day 9 by monitoring NO-loaded docetaxel nanomedicines by DLS, respectively. distributed. The results showed that the particle size and particle size distribution index of the NO-loaded docetaxel nanomedicine did not change significantly during the detection period, indicating that it has good stability. figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com