Thioglucose spiroketal derivative and use thereof as therapeutic agent for diabetes
A technology of drugs and compounds, applied in the field of glucoglucosinospirit derivatives, which can solve problems such as difficult to achieve stable effects and large individual differences
Inactive Publication Date: 2009-09-02
CHUGAI PHARMA CO LTD
View PDF29 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, in the case of administering a prodrug, it is desired to properly metabolize it in or near the target organ and convert it into an active compound. achieve a stable effect
In addition, attempts have been made to convert the glycosidic bond of the compound into a carbon-carbon bond (see Patent Documents 4 to 21), and to convert the glucose moiety into 5-thioglucose (see Patent Documents 22 to 26), but there are no active In terms of drug properties and metabolic stability, it needs to be further improved
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
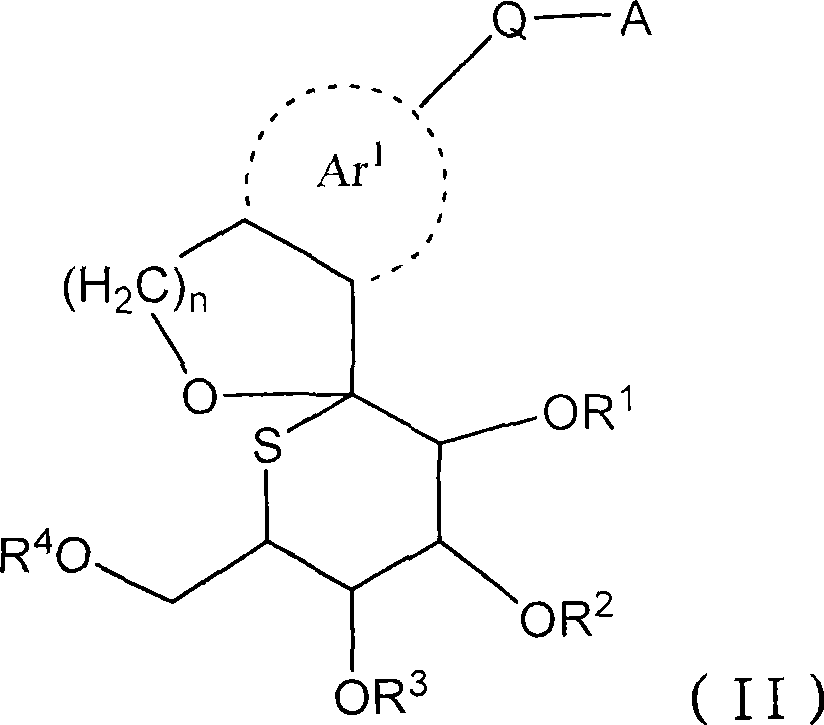
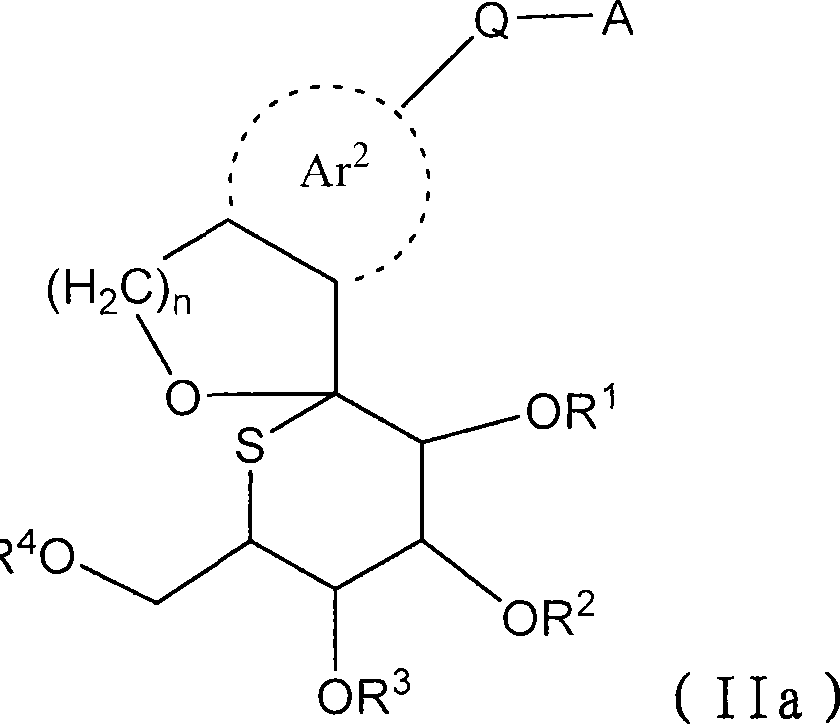
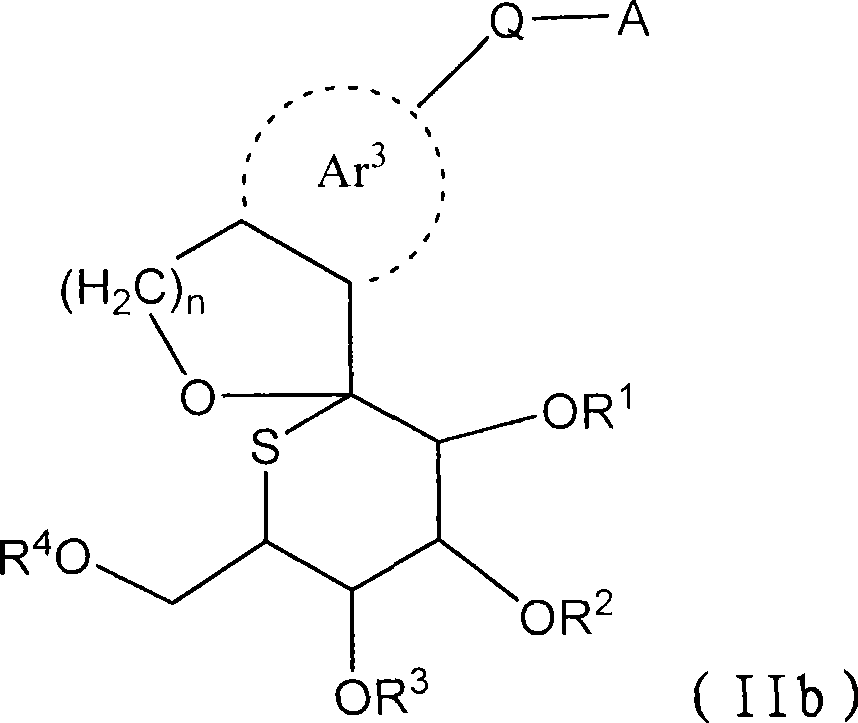
The present invention provides a compound represented by the formula (II), a prodrug thereof, a pharmaceutically acceptable salt of the compound or the prodrug, a pharmaceutical agent and a pharmacologically composition each comprising the compound, and others. (II) wherein R, R, R and R is independently selected from a hydrogen atom, a C1-C6 alkyl group which may be substituted, a C7-C14 aralkyl group which may be substituted and -C(=O)Rx; Rx represents a C1-C6 alkyl group which may be substituted, an aryl group which may be substituted, a heteroaryl group which may be substituted, a C1-C6 alkoxy group which may be substituted, or -NReRf; Ar represents an aromatic carbon ring which may be substituted and which may be fused, or an aromatic heterocyclic ring; Q represents -(CH2)m-(L)p- or -(L)p-(CH2)m-; m represents an integer selected from 0 to 2; n represents an integer selected from 1 and 2; p represents an integer selected from 0 and 1; L represents -O-, -S- or -NR-; and A represents an aryl group which may be substituted or a heteroaryl group which may be substituted.
Description
technical field [0001] The present invention relates to sulfglucospiro derivatives, their drug prodrugs and their pharmacologically acceptable salts which can be used as medicine. The present invention is particularly concerned with the ability to inhibit Na + -Glucose co-transporter 2 (SGLT2), which can be used as insulin-dependent diabetes (type 1 diabetes), non-insulin-dependent diabetes (type 2 diabetes), diabetes, diabetic complications, obesity, etc. caused by hyperglycemia Sulfatose spiro derivatives, prodrugs thereof, and salts thereof for use as prophylactic or therapeutic drugs for the disease. Background technique [0002] Diabetic patients are increasing in recent years due to Westernization of eating habits and long-term lack of exercise. In diabetic patients, decreased insulin secretion and decreased sensitivity to insulin due to chronic hyperglycemia can be observed, which further increase blood sugar levels, thereby exacerbating symptoms. Currently, biguan...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07H7/06A61K31/7048A61P3/04A61P3/06A61P3/10A61P9/04A61P9/10A61P9/12A61P13/12A61P19/06A61P25/00A61P27/02A61P31/04A61P43/00
CPCC07H7/06A61P13/12A61P19/06A61P25/00A61P27/02A61P3/04A61P31/04A61P3/06A61P43/00A61P9/04A61P9/10A61P9/12A61P3/10A61K31/7048
Inventor 佐藤勉
Owner CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com