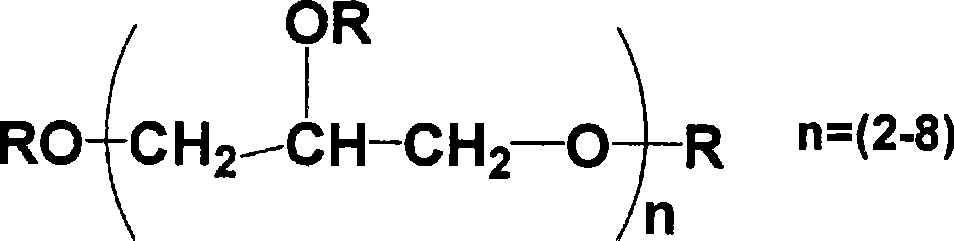
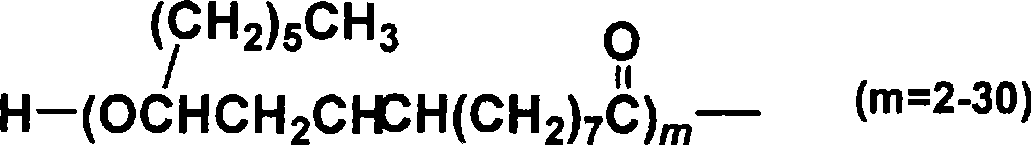W/o/w emulsion composition
A composition and emulsion technology, which is applied in the direction of drug combination, active ingredient of heterocyclic compounds, emulsion delivery, etc., can solve problems such as difficult injection of W/O/W emulsion, difficult emulsification of W/O droplets, increase in viscosity of injection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-13
[0073] Preparation of W / O / W Emulsion Composition
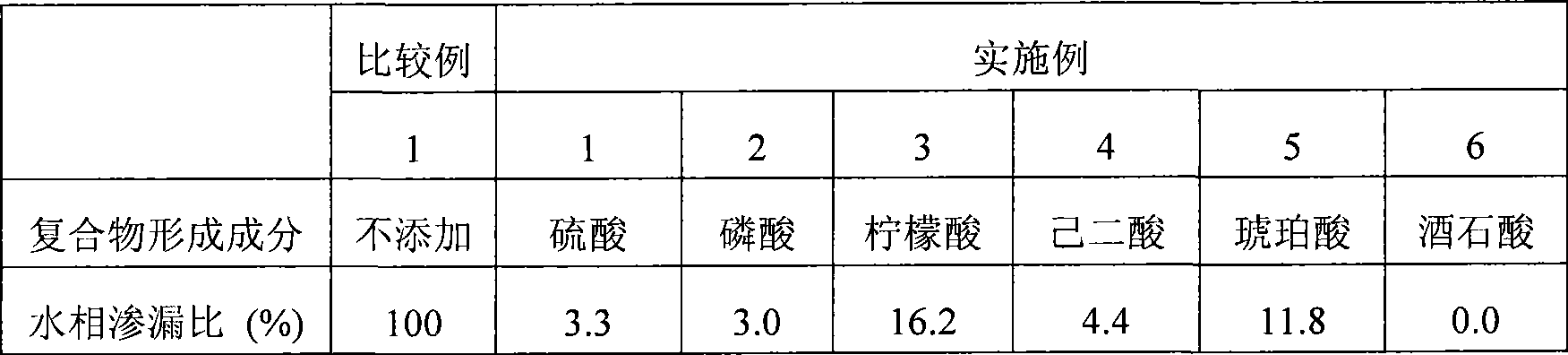
[0074] Using epirubicin hydrochloride as a drug ingredient and each compound shown in Table 1 as described below as a complex forming ingredient, a compound containing 0.1w / v% epirubicin hydrochloride dissolved in water for injection, 1w / v% Aqueous solution of complex forming component, 4.75w / v% glucose and 0.014M citric acid for internal aqueous phase.
[0075] Polyglyceryl-condensed-ricinoleate (PGCR) (i.e., lipophilic surfactant) was dissolved in ethyl ester of iodinated poppy seed oil fatty acid (trade name: Lipiodol (Guerbet)) ( That is, in the oily component), 5.5 mL of an oily phase having a PGCR concentration of 10 w / v% was prepared. Then, 1 mL of the above-mentioned aqueous solution for the inner aqueous phase was added thereto, and stirred at 25000 rpm for 10 minutes with a Polytron homogenizer (manufactured by Kinematica) under nitrogen flow and heating at 50°C. Thus, an emulsion was prepared.
[0076]Simultane...
Embodiment 14-20
[0086] Preparation of W / O / W Emulsion Composition
[0087] Using doxorubicin hydrochloride as a drug ingredient and each compound as shown in Table 2 below as a complex-forming ingredient, W / O / W emulsion compositions were prepared by the production methods used in Examples 1-13.
[0088] Determination of Water Phase Leakage Ratio
[0089] The aqueous phase leakage ratio (%) was determined by the method used in Examples 1-13.
[0090] Table 2
[0091] Pharmaceutical ingredients: Adriamycin hydrochloride
[0092]
[0093]
Embodiment 21
[0095] Preparation of W / O / W Emulsion Composition
[0096] W / O / W emulsion compositions were prepared by the production methods used in Examples 1-13 using bupivacaine hydrochloride as a drug ingredient and each compound as shown in Table 3 below as a complex-forming ingredient.
[0097] Determination of Water Phase Leakage Ratio
[0098] The aqueous phase leakage ratio (%) was determined by the method used in Examples 1-13. Drug concentrations were calculated from absorbance.
[0099] table 3
[0100] Drug ingredient: bupivacaine hydrochloride
[0101]
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com