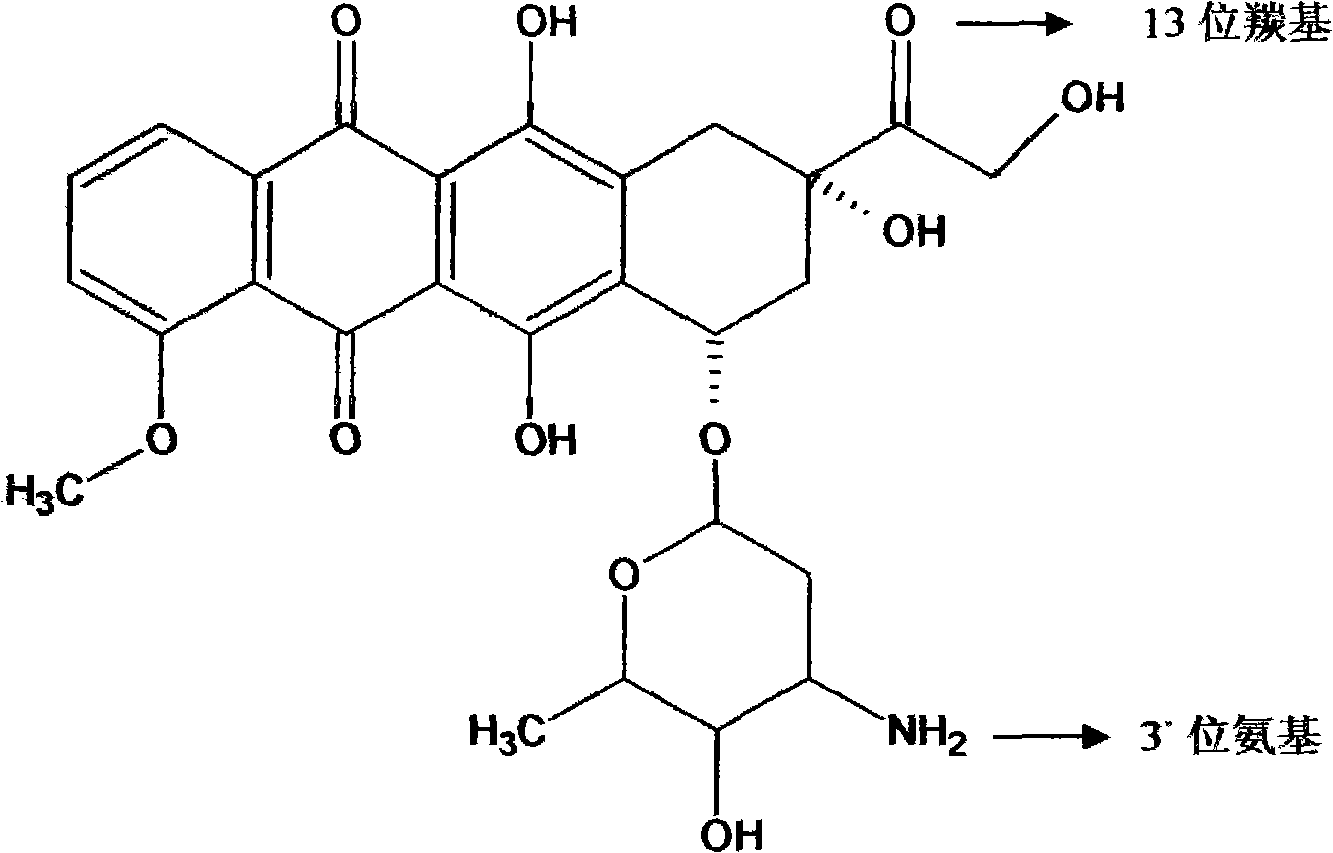
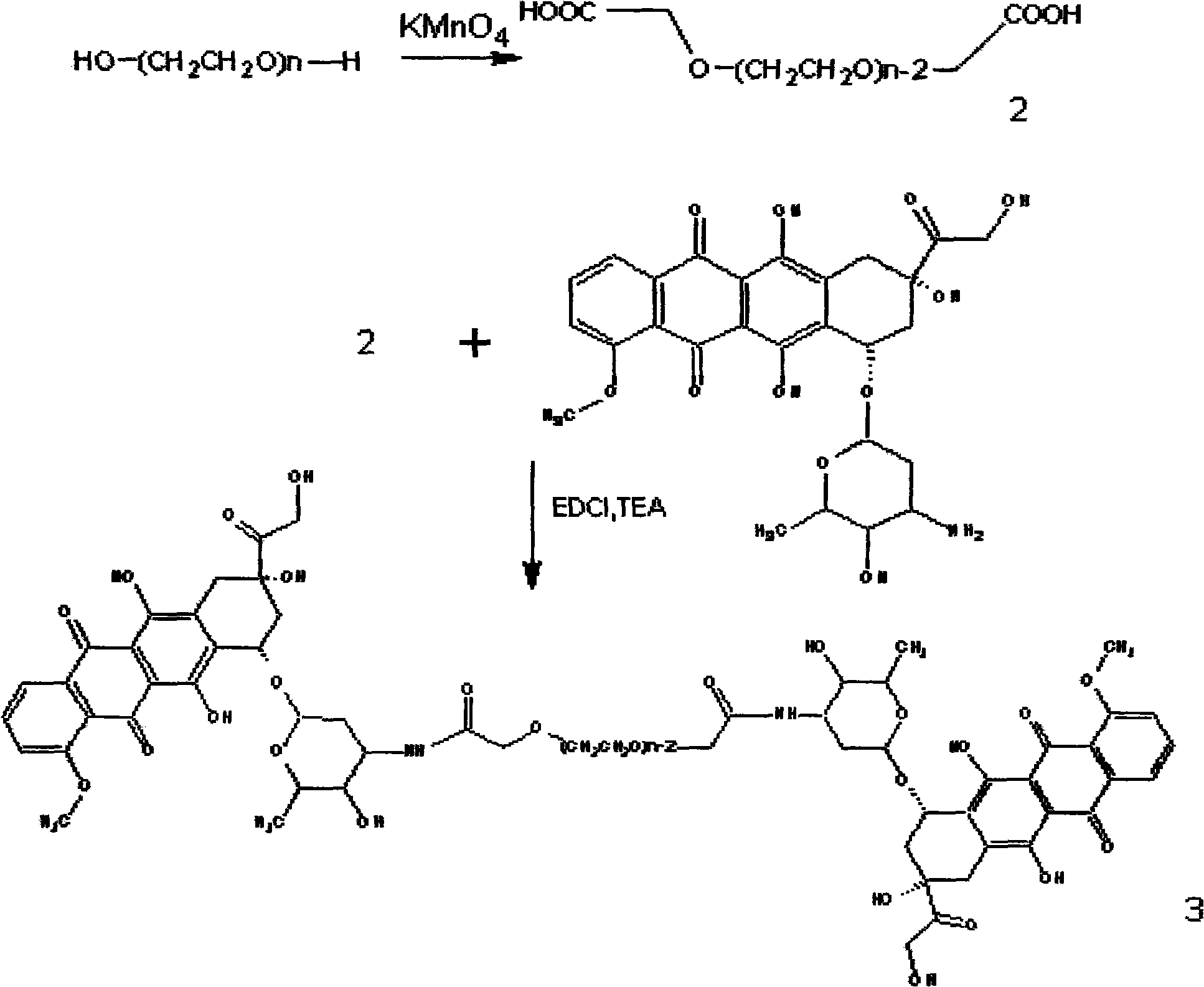
Preparation method for amycin pro drug and application thereof
A prodrug, doxorubicin technology, applied in the field of preparation of doxorubicin prodrug, can solve the problems of tumor cell drug resistance, cytotoxicity limitation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
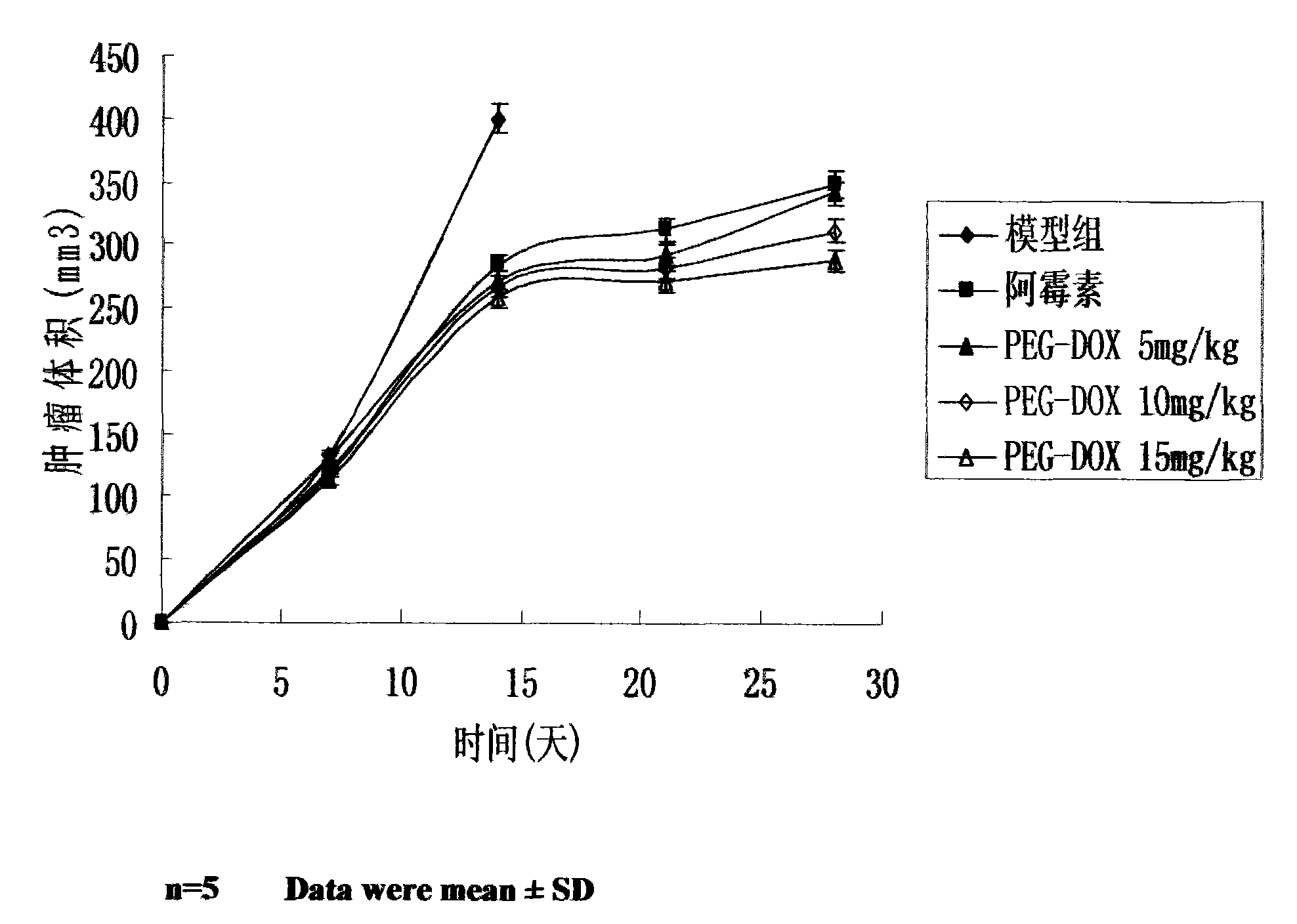
[0017] Study on Antitumor Activity of Doxorubicin Prodrugs in Vitro
[0018] 1. Purpose:
[0019] The anti-tumor activity of the prodrug was evaluated by in vivo and in vitro experiments with doxorubicin as a positive control.
[0020] 2 Research methods:
[0021] 2.1 In vitro cytotoxicity evaluation of prodrugs
[0022] Dissolve various drugs to be tested in serum-free culture medium (DMSO less than 0.1%) according to the set concentration, and use 0.25% trypsin to digest the liver cancer cells HepG-2 and breast cancer cells that have grown to the logarithmic growth phase. Cells MCF-7 and breast cancer cells MDA-MB-231 were seeded in 96-well plates at a density of 1×10 4 After culturing for 24 hours, DOX, DOX-LNA, PEG-DOX amid, PEG-DOX hydrazone (the serum-free culture medium) were added respectively at 0.001, 0.01, 0.1, 1, 10, and 100 μM (concentrations of doxorubicin) After 48 hours of incubation, add 20 μL MTT and continue to incubate for 4 hours, discard the culture m...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap