Method for preparing montelukast sodium salts
一种孟鲁司特钠、胺盐的技术,应用在钠有机化合物、呼吸系统疾病、有机化学等方向,能够解决正丁基锂存在危险性、总收率降低、昂贵试剂等问题,达到简化偶联和水解反应、提高效率、高收率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
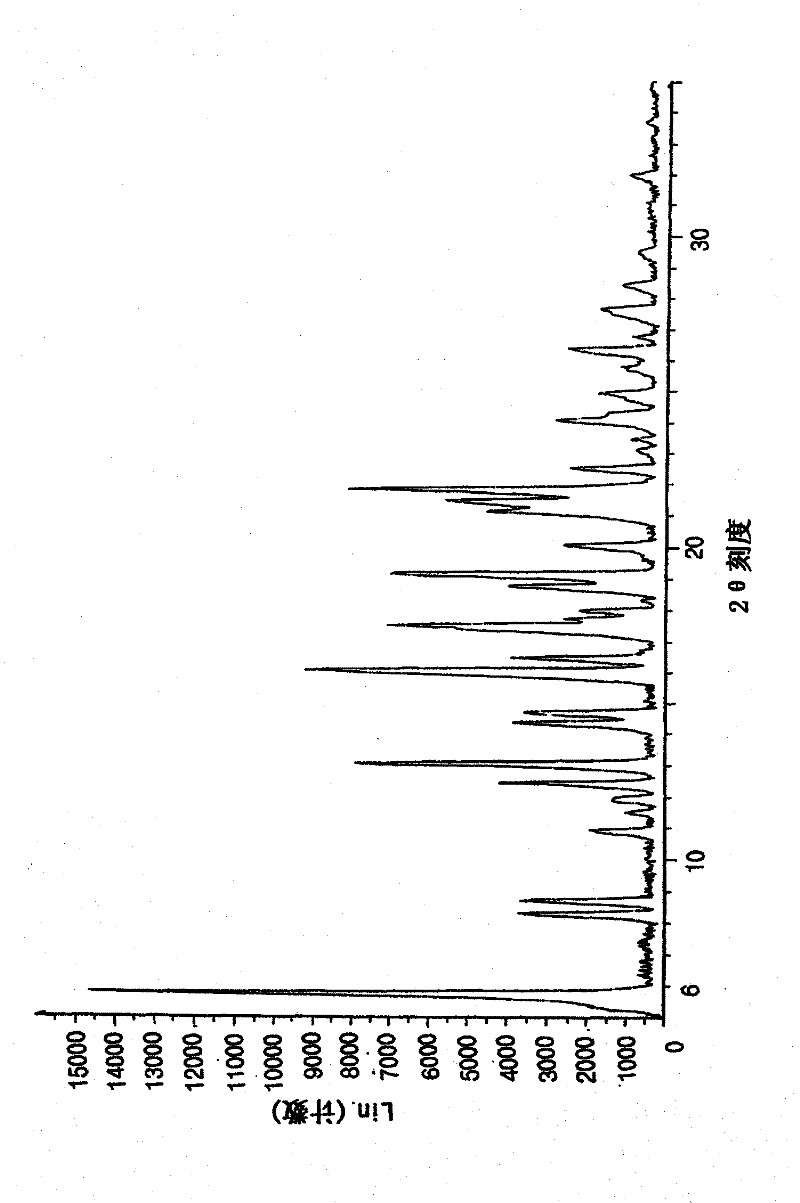
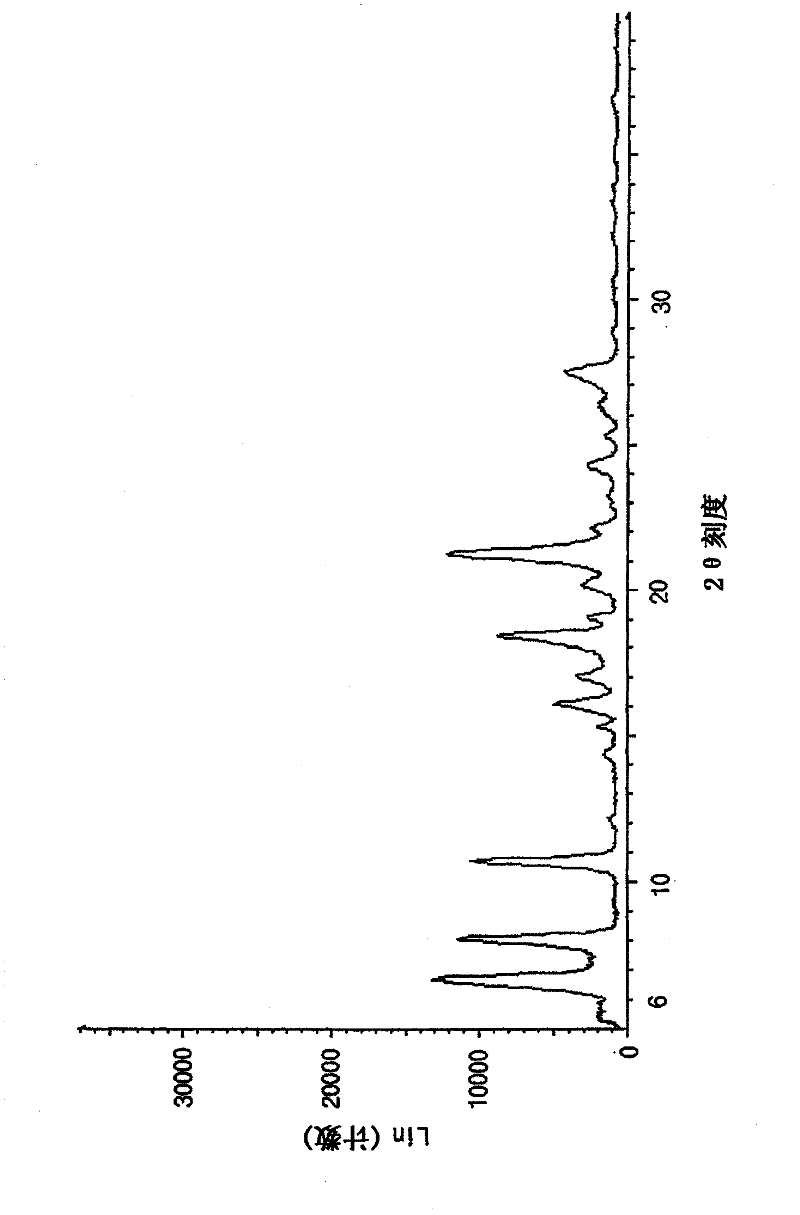
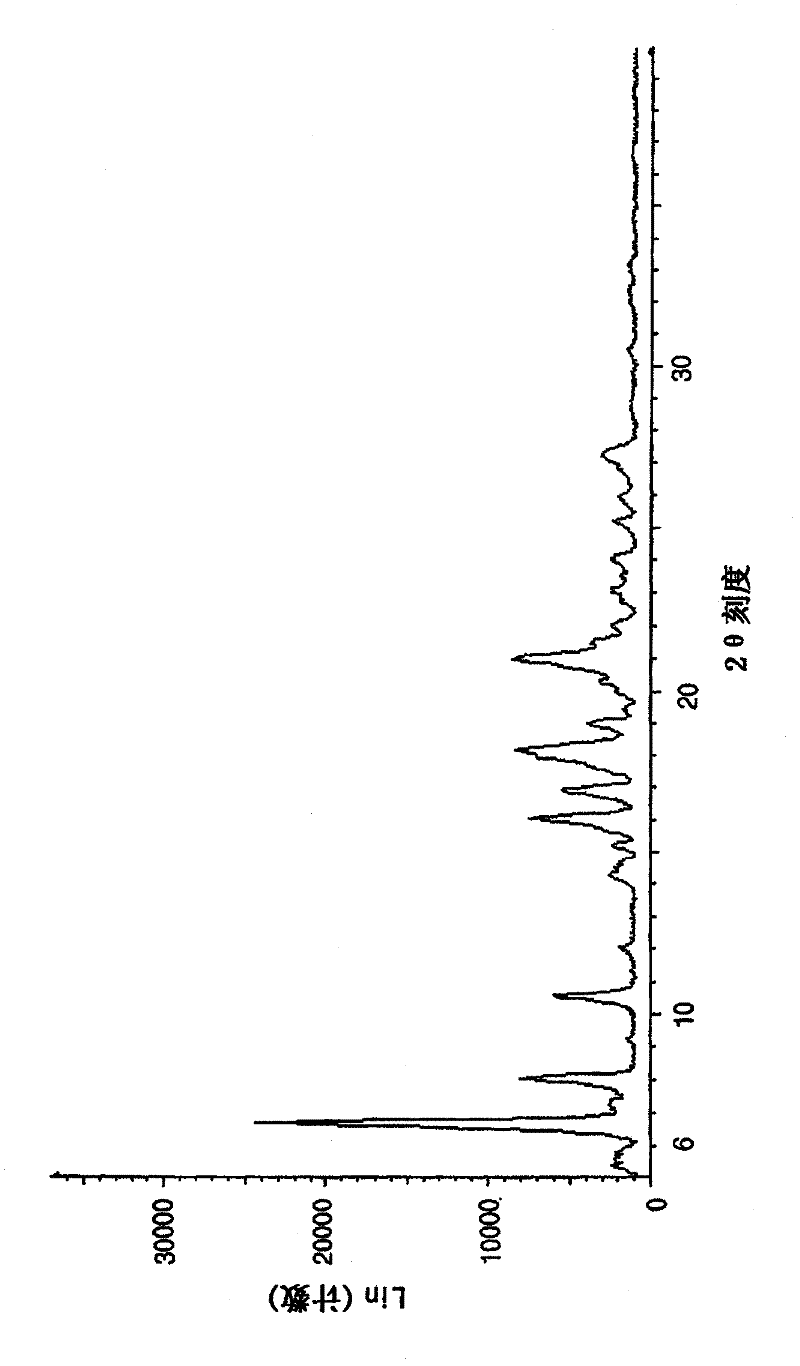
Image
Examples
Embodiment 1
[0052] Preparation of 2-(2-(3(S)-(3-(7-chloro-2-quinolyl)-vinyl)phenyl)-3-methylsulfonyloxypropyl)phenyl-2-propane Alcohol (2)
[0053] 2-(2-(3(S)-(3-(7-chloro-2-quinolyl)-vinyl)phenyl)-3-hydroxypropyl)phenyl-2-propanol (20.0g ) was dissolved in 70 ml of tetrahydrofuran, the temperature of the reactor was lowered to 0° C., and diisopropylethylamine (8.43 ml) was slowly added dropwise thereto. The internal temperature of the reactor was lowered to -25°C, methanesulfonyl chloride (5.54 g) was slowly added dropwise under nitrogen, and the reaction mixture was stirred at -25°C for one hour. After completion of the reaction, 200 ml of acetonitrile was slowly added dropwise at an internal reactor temperature below -20°C. The resulting solid was filtered using a cryofilter under nitrogen, washed with 100 ml of acetonitrile cooled below 0°C and dried to give 21.7 g of the title compound as a pale yellow solid.
[0054] 1 H NMR (400MHz, CDCl 3 ): δ8.12(m, 2H), 7.75(m, 3H), 7.66(d,...
Embodiment 2
[0056] Preparation of [R-(E)]-1-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methyl ethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid (4)
[0057] Methyl 1-(mercaptomethyl)cyclopropaneacetate (1.37 g) was dissolved in 15 ml tetrahydrofuran and the internal reactor temperature was cooled to -15°C. Sodium bistrimethylsilylamide (2M solution in tetrahydrofuran, 4.8 ml) was added dropwise to the reaction mixture under nitrogen, followed by stirring at -15°C for one hour. At this reaction temperature, the compound (4.0 g) prepared in Example 1 was added thereto, followed by stirring for 7 hours. After the coupling reaction was completed, the internal reactor temperature was raised to room temperature, and a 10% lithium hydroxide aqueous solution (12 g) was added thereto. The internal reactor temperature was raised to 50°C and reacted at this temperature for 10 hours. After the reaction was completed, the reaction solution was cooled to room temperature,...
Embodiment 3~8
[0059] The reaction was carried out in the same manner as in Example 2 except for changing the types of solvent and base. The results thus obtained are shown in Table 1 below.
[0060] Table 1
[0061]
solvent
alkali
Yield (%)
Example 3
Tetrahydrofuran
Sodium Bistrimethylsilylamide (95%)
84.7
Example 4
Tetrahydrofuran
Lithium bistrimethylsilylamide (95%)
68.7
Example 5
Tetrahydrofuran
Potassium Bistrimethylsilylamide (95%)
84.5
Example 6
dimethylformamide
Sodium Bistrimethylsilylamide (95%)
82.1
[0062] Example 7
dimethylformamide
Lithium bistrimethylsilylamide (95%)
83.5
Example 8
3-Methyltetrahydrofuran
Potassium Bistrimethylsilylamide (95%)
66.4
[0063] * 95%: the purity of the reactant
[0064] As can be seen from above Table 1, according to the method of the present invention, by using bistrimethylsilylamino alka...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap