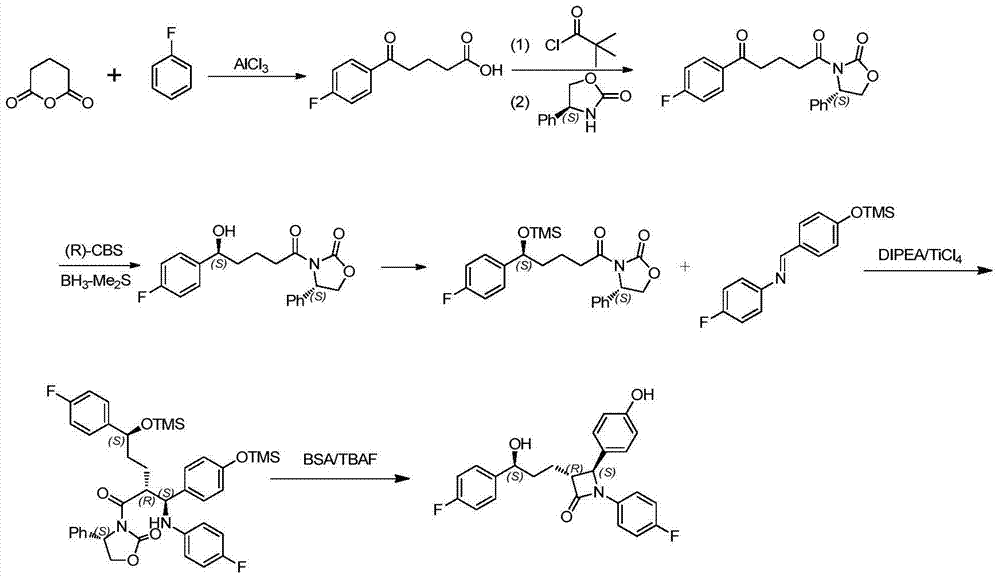
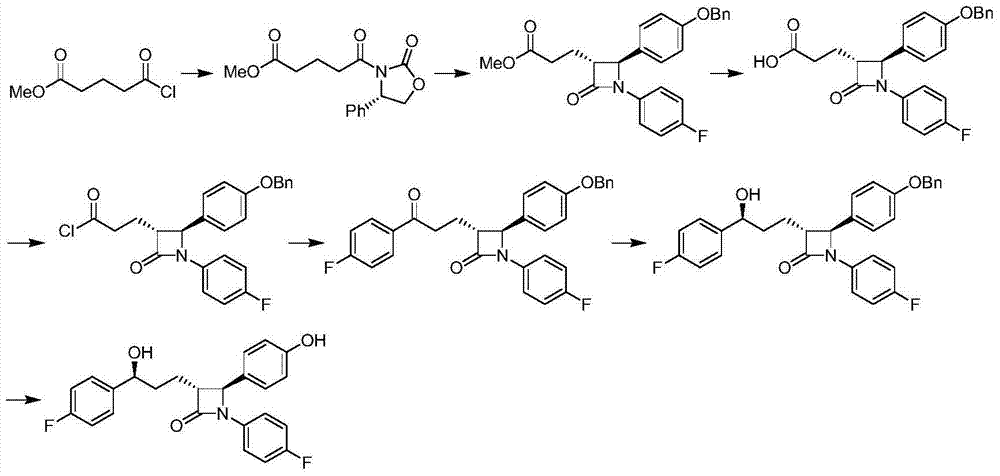
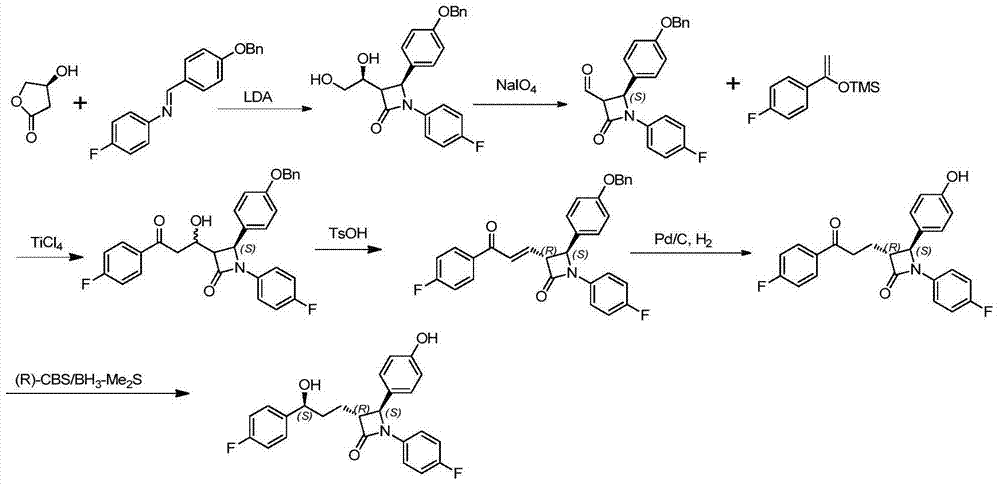
Ezetimibe synthesis method and Ezetimibe intermediate synthesis method
A technology for ezetimibe and a synthesis method, which is applied in the field of small molecule chemical drug preparation, can solve the problems of high solvent toxicity, harsh reaction conditions, low yield and purity, and achieves easy control of process conditions, mild reaction conditions, and high product quality. high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of compound (2)
[0048]
[0049] Compound (1) (10g, 81.90mmol) and p-fluoroaniline (9g, 81.00mmol) were dissolved in isopropanol (75ml), heated to 50°C, stirred for 1h, cooled to room temperature, suction filtered, and isopropanol ( 10ml) to obtain a pale yellow solid, compound (2) (14.2g, 80% yield).
Embodiment 2
[0051] Preparation of compound (3)
[0052]
example 2-1
[0053] Example 2-1: Preparation of compound (3a) (ie, R=TBS in compound (3))
[0054] Compound (2) (6g, 27.88mmol), TBDMSCl (5.04g, 33.44mmol) and imidazole (3.8g, 55.80mmol) were dissolved in DMF (12ml), heated to 60°C, stirred for 4h, cooled to room temperature, added water (60ml), add ethyl acetate (20ml×3) for extraction, combine the organic phases, wash the organic phase with water (20ml×2), add MgSO to the organic phase 4 Drying and concentration gave a pale yellow solid, compound (3a) (9.0 g, 97.5% yield).
[0055] 1 H-NMR (CDCl 3 ,400MHz,δppm):8.18(s,1H),7.80(d,J=8.8,2H,),7.19(m,2H),7.12(m,2H),6.95(d,J=8.8,2H), 1.05(s,9H),0.22(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com