Bioabsorbable polymeric medical device
A biological, locking device technology, used in medical science, stents, prostheses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
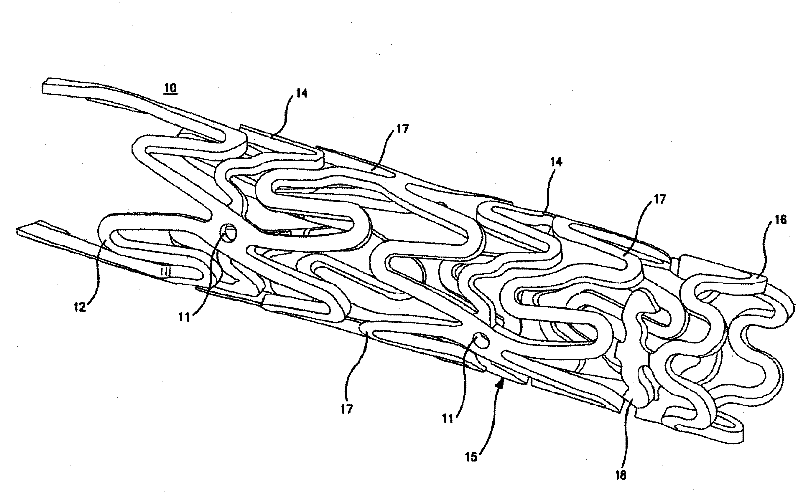
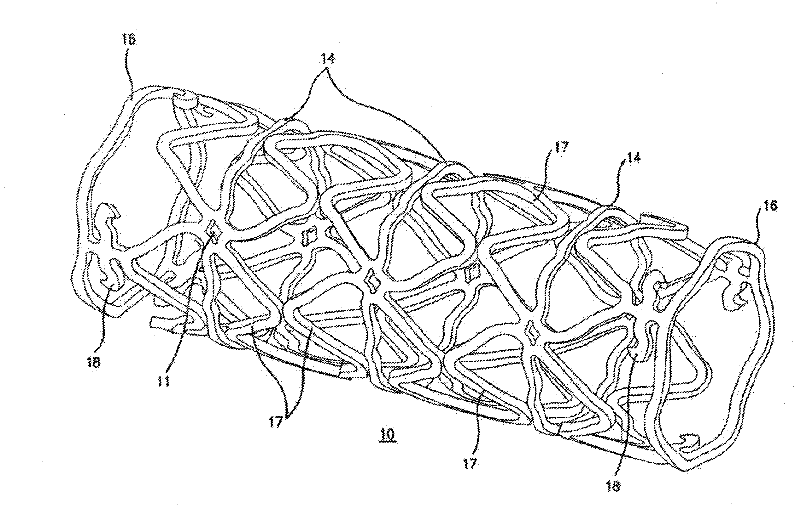
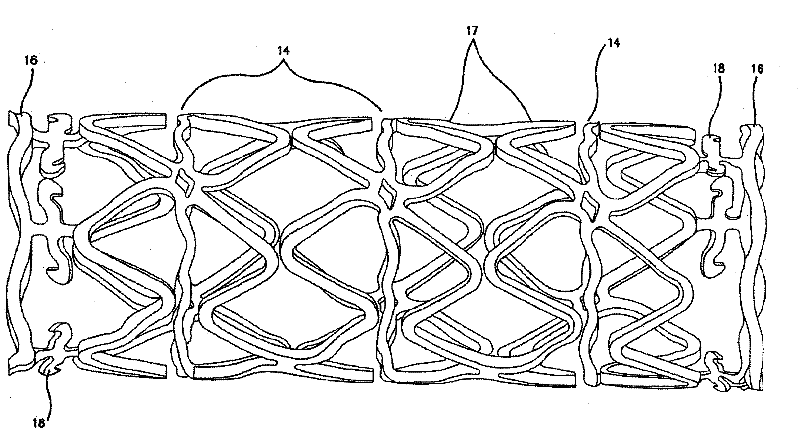
[0102] Disclosed herein are novel structural elements and novel compositions that can be used to prepare such novel structural elements. Embodiments of the present invention are useful in the treatment of a variety of diseases and physiological conditions.
[0103] In recent years, metal stents have been used to help clear clogged lumens of the vasculature. However, certain adverse results have reduced the effectiveness of metal stent grafts in arterial vessels. For example, because such stents have been shown to stimulate the tendency to form scar tissue or restenosis in wounds in the region of the vessel in which they are placed. This effect becomes more deleterious with treatments using small diameter tubing. In addition, it is important to avoid damage to the arterial wall during stent insertion. The purpose of these factors, although somewhat difficult to control in the first case, is to attempt to reduce the mechanical causes of excess clot and scar formation in the v...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap