Nanometer micelle capable of intelligently releasing medicine as well as preparation method and application thereof
A nano-micelle and drug-loaded nano-technology, which is applied in the fields of polymer chemistry and biomedical engineering, can solve the problems that the particle size of the micelles is easy to be swallowed, easy to disintegrate, and the assembly is easy to disintegrate, so as to facilitate cell absorption and good chain flexibility, prolonging the blood circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of nanomicelle PEG-PAsp(MEA)-PAsp(DIP)
[0068] 1. Preparation of polymer PEG-PBLA:
[0069] The polymer is PEG-NH 2 As an initiator, it can be obtained by ring-opening polymerization of BLA-NCA. 0.8 g PEG-NH 2 (0.4mmol, 2000 g / mol) was added to a 100 mL reaction flask and dissolved in 50 mL of anhydrous dichloromethane. Weigh 1.0 g of BLA–NCA (4 mmol, 249.22 g / mol), add 5 mL of anhydrous DMF (N,N-dimethylformamide) to dissolve. Transfer the dissolved BLA–NCA solution into the PEG-NH 2 In a reaction flask, the reaction was stirred at 35°C for 72h. After the reaction, the reaction solution was dropped into excess cold ether (eg 500ml) for precipitation, and the final product was obtained by suction filtration, repeated washing with anhydrous ether, and vacuum drying.
[0070] 2. Polymer PEG-PBLA-COCH 2 Preparation of Br:
[0071] Weigh 0.9 g PEG-PBLA (3025 g / mol, 0.3 mmol) in a 25 mL reaction vial, add 15 mL CHCl 3 To dissolve, then add 0...
Embodiment 2
[0089] Example 2 Preparation of drug-loaded nanomicelles
[0090] 5mg PEG-PAsp(MEA)-PAsp(DIP) polymer and 1mg DOX were co-dissolved in a mixed solvent of THF and DMSO (1mL, v / v=1 / 1), and triethylamine was added to adjust the pH value of the solution to 10, At the same time, DTT (10 eq in the polymer) was added to reduce the disulfide bonds present in the polymer for 30 min. Under ultrasonic conditions, the above solution was slowly dripped into the PBS (pH 7.4) solution, and the resulting mixture was passed through oxygen for 2 hours, then placed in a dialysis bag (MWCO: 1000 Da), and passed through in the PBS (pH 7.4) solution Dialyzed under oxygen for 2 days to obtain drug-loaded nanomicelles.
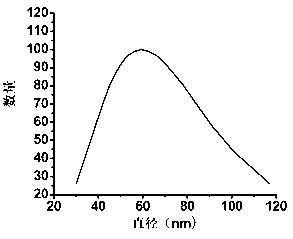
[0091] The particle size of the obtained drug-loaded nanomicelles is measured by a dynamic light scattering system, and the test results are shown in figure 2 . From the dynamic light scattering parabola diagram, it can be seen that the average particle size of the drug-loaded ...
Embodiment 3
[0092] Example 3 Preparation of loaded nano-gold nano-binding gel
[0093] Take 1 mL of the nanomicelle solution (0.4 mg / mL, pH 7.4) prepared in Example 1, add 20 μL of HauCl 4 (1mg / mL), adjust and maintain the pH value at 7.4, stir for 15min, then add 20μL of hydroxylamine (50wt%), and continue stirring for 15min to obtain.
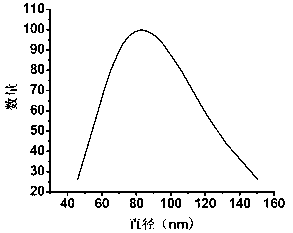
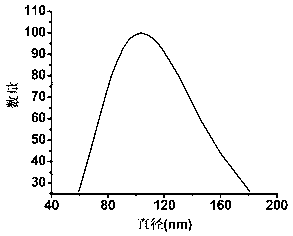
[0094] The particle size of the nano-micelle loaded with nano-gold was measured by a dynamic light scattering system, and its shape was determined by observation with a transmission electron microscope. The test results are shown in image 3 with Figure 7 . It can be seen from the dynamic light scattering parabola diagram that the average particle size of nano-gold modified micelles is 103.6nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com