Antibodies that specifically bind to a beta oligomers and use thereof
An antibody, tetramer technology, applied in the direction of antibodies, specific peptides, drug combinations, etc., can solve the problems of side effects, difficult to measure Aβ oligomers alone, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
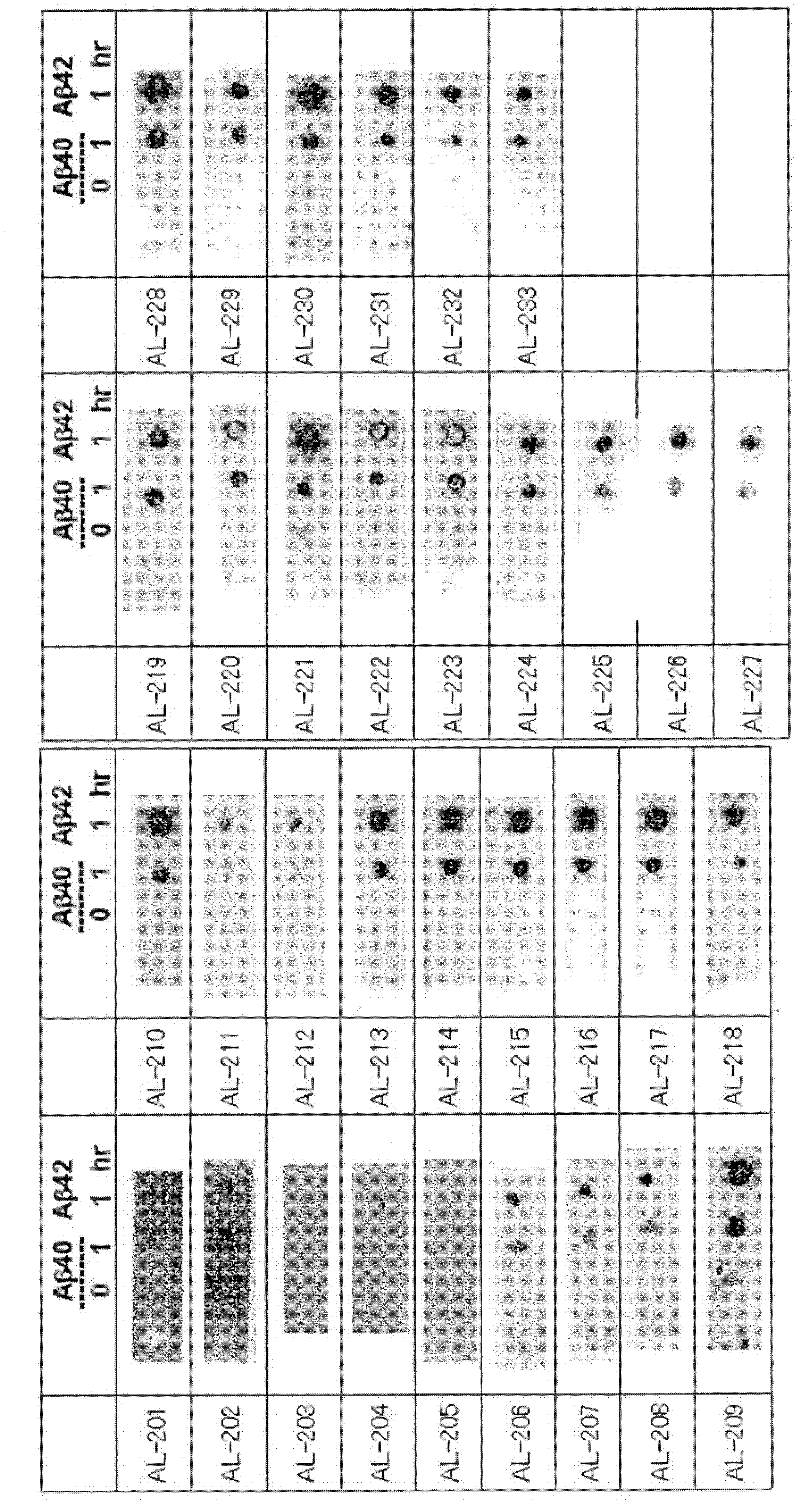
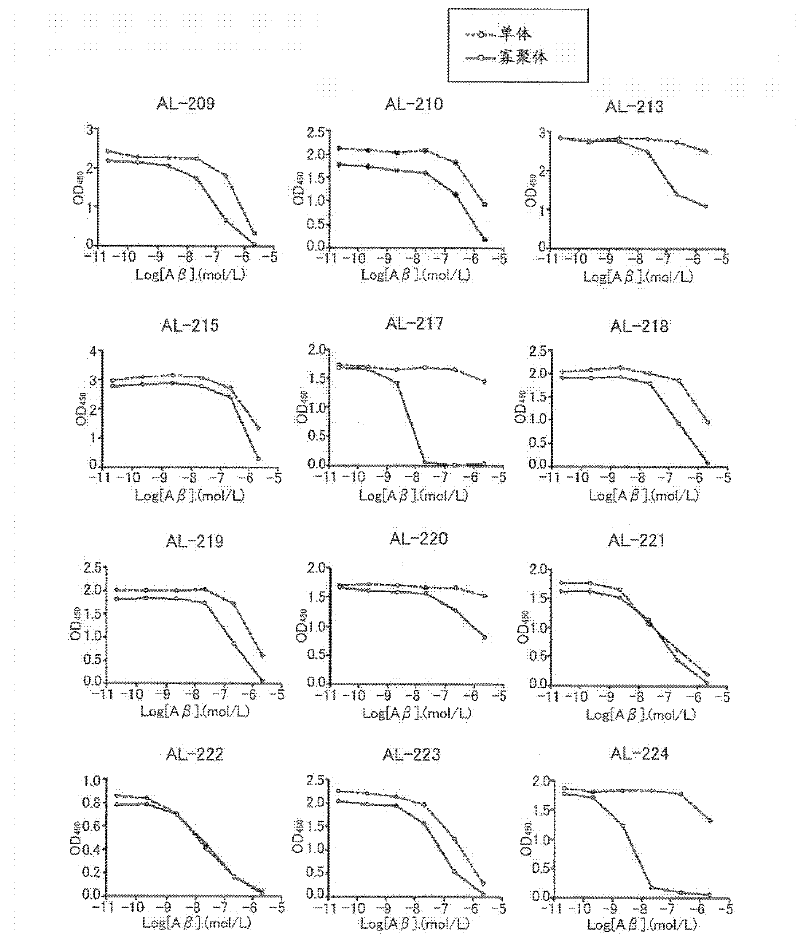
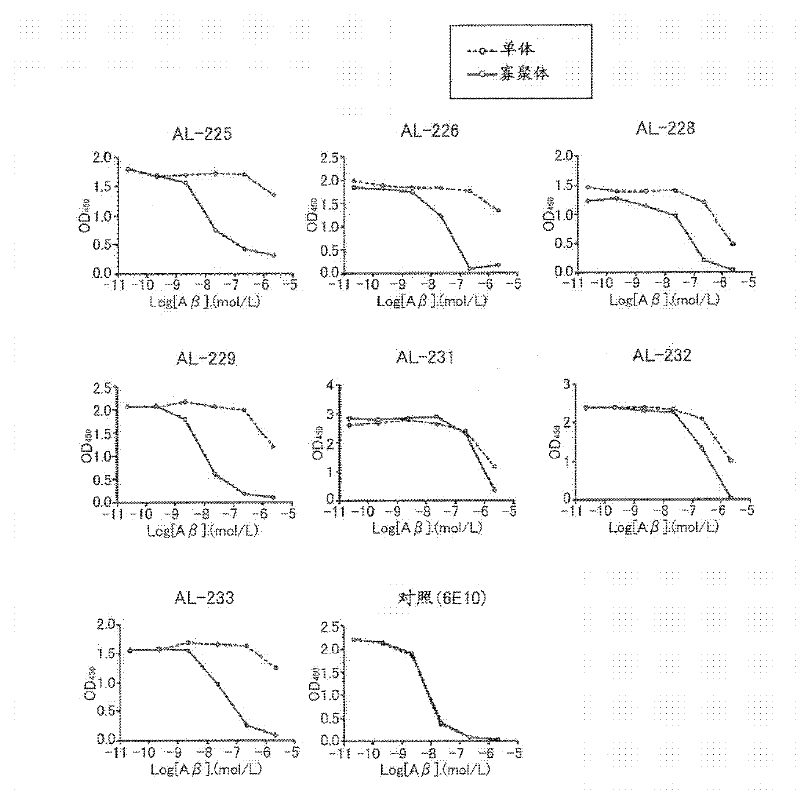
[1373] Hereinafter, the present invention is specifically described with reference to examples, but should not be construed as being limited thereto.
[1374] method :
[1375] Antigen preparation
[1376] Synthetic Aβ1-42 (Peptide Institute, Inc., Osaka) was dissolved in distilled deionized water or 10 mM phosphate buffer, and incubated at 37°C for 18 hours. Peptides were then resolved using NuPAGE 4-12% Tris-Glycine Gel SDS-PAGE, and after visualization by CBB staining, only Aβ1-42 tetramers not contaminated with Aβ1-42 monomers were excised. Antigens were prepared by finely crushing the tetramer-containing gel or by extracting the tetramer from the gel.
[1377] Preparation of antibody-producing hybridomas
[1378] BALB / c mice were immunized by injecting the antigen prepared by the method described above into their footpads or abdominal cavity. Then, booster immunizations are given six times. Hybridomas were prepared from inguinal lymph node cells or splenocytes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com