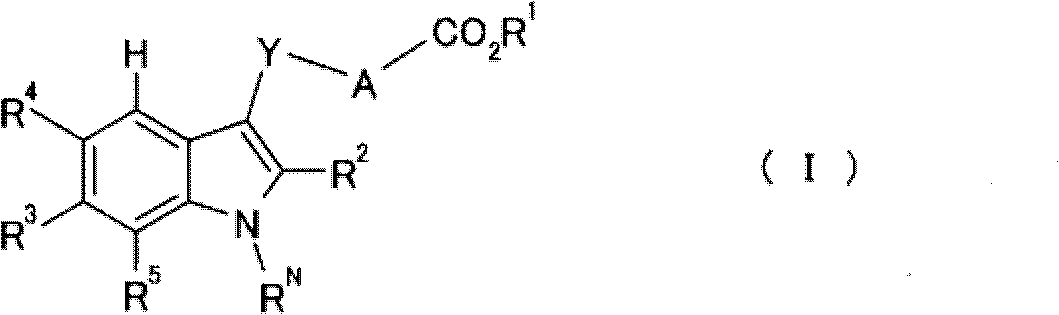
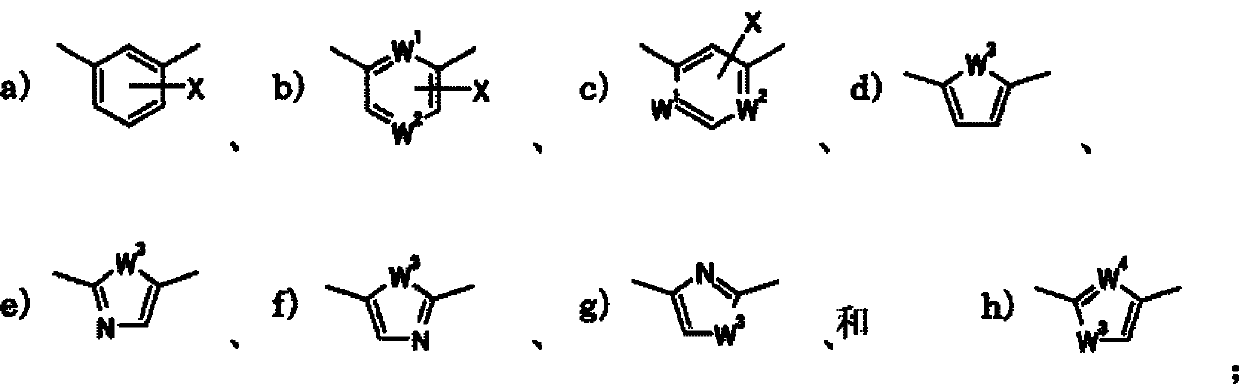
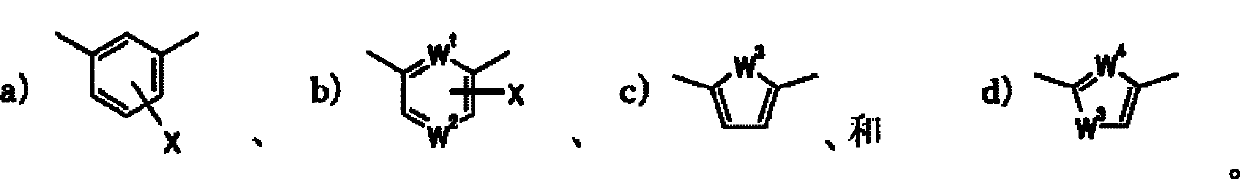Indole derivative and pharmacologically acceptable salt thereof
A kind of technology of pharmaceutically acceptable salt and compound, applied in the field of indole derivatives or pharmaceutically acceptable salts thereof, can solve the problems such as no suggestion or disclosure of receptor antagonism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0143] Preferred embodiments of the present invention are as follows:
[0144] Y is methylene;
[0145] A is a benzene ring or a pyridine ring;
[0146] R 1 is a hydrogen atom;
[0147] R 2 is a phenyl group or a 5-membered aromatic heterocyclic group;
[0148] R 3 is methoxy or ethoxy; and
[0149] R N is a hydrogen atom.
[0150] Examples of specific compounds encompassed within this embodiment include the following compounds:
[0151] 6-(6-methoxy-2-phenyl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (Example 4-1), 3-(6-methoxy-2-benzene Base-1H-indol-3-ylmethyl)benzoic acid (Example 4-2), 3-(6-methoxy-2-thiophen-3-yl-1H-indol-3-ylmethyl ) benzoic acid (embodiment 4-12), 6-(6-ethoxy-2-phenyl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (embodiment 4-34), 6 -(6-methoxy-2-thiophen-3-yl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (Example 11-1), 6-[2-(3-fluorobenzene Base)-6-methoxy-1H-indol-3-ylmethyl]pyridine-2-carboxylic acid (Example 11-5), 6-(2-furan-3-...
Embodiment approach 2
[0153] Another preferred embodiment of the present invention is as follows:
[0154] Y is methylene;
[0155] A is a benzene ring or a pyridine ring;
[0156] R 1 is a hydrogen atom;
[0157] R 2 is unsubstituted phenyl;
[0158] R 3 is methoxy; and
[0159] R N is a hydrogen atom.
[0160] Examples of specific compounds encompassed within this embodiment include the following compounds:
[0161] 6-(6-methoxy-2-phenyl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (Example 4-1), 3-(6-methoxy-2-benzene Base-1H-indol-3-ylmethyl)benzoic acid (Example 4-2), 2-fluoro-3-(6-methoxy-2-phenyl-1H-indol-3-ylmethyl) Base) benzoic acid (Example 16-9), 6-(6-methoxy-5-methyl-2-phenyl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (Example 16-12), 6-(5-chloro-6-methoxy-2-phenyl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (embodiment 16-13), 6-( 5-fluoro-6-methoxy-2-phenyl-1H-indol-3-ylmethyl)pyridine-2-carboxylic acid (Example 16-16), and 2-fluoro-5-(6-methyl Oxy-2-phenyl-1H-i...
Embodiment approach 3
[0163] Another preferred embodiment of the present invention is as follows:
[0164] Y is methylene;
[0165] A is a benzene ring or a pyridine ring;
[0166] R 1 is a hydrogen atom;
[0167] R 2 is a substituted phenyl group;
[0168] R 3 is methoxy; and
[0169] R N is a hydrogen atom.
[0170] Examples of specific compounds encompassed within this embodiment include the following compounds:
[0171] 6-[2-(3-fluorophenyl)-6-methoxy-1H-indol-3-ylmethyl]pyridine-2-carboxylic acid (Example 11-5), 6-[2-(4 -fluorophenyl)-6-methoxy-1H-indol-3-ylmethyl]pyridine-2-carboxylic acid (Example 11-13), 6-[2-(3-chlorophenyl)-6 -Methoxy-1H-indol-3-ylmethyl]pyridine-2-carboxylic acid (Example 11-21), 6-[6-methoxy-2-(3-methoxyphenyl)- 1H-indol-3-ylmethyl]pyridine-2-carboxylic acid (Example 11-34), 6-[2-(3,4-difluorophenyl)-6-methoxy-1H-indole -3-ylmethyl]pyridine-2-carboxylic acid (Example 16-2), 6-[6-methoxy-2-(3,4,5-trifluorophenyl)-1H-indole-3 -ylmethyl]pyridine-2-carboxylic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com