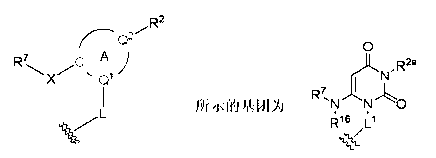
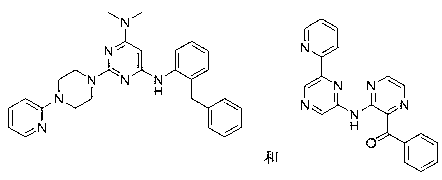
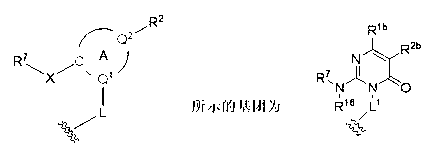
Novel heterocyclic derivatives and pharmaceutical composition containing same
A composition and medicine technology, applied in the field of novel heterocyclic derivatives and pharmaceutical compositions containing the same, can solve the problems of no record of analgesic receptor antagonism, no record of receptor antagonism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1843] Preparation of 1-(4-chlorobenzyl)-3-ethylamino-6-(4-isopropoxyphenylamino)benzene (I-071)
[1844] [chemical 120]
[1845]
[1846] To a mixture of 3-bromo-4-fluoro-1-nitrobenzene (1.0 g, 4.6 mmol) and DMSO (5 mL), potassium carbonate (1.01 mg, 7.3 mmol) and 4-isopropoxyaniline ( 1.03g, 6.8mmol), stirred at 100°C for 0.5 hours. Water (20 mL) was added to the reaction solution, followed by extraction with ethyl acetate (30 mL×2). The extract was washed with saturated brine (20 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (ethyl acetate / hexane), and the obtained target product was solidified with ethyl acetate and hexane to obtain 3-bromo-4-(4-isopropoxy Phenylamino)-1-nitrobenzene (0.55g, yield: 35%).
[1847] 1H-NMR(δppmTMS / DMSO-d6):1.28(6H,d,J=6.0Hz),4.61(1H,sept,J=6.0Hz),6.76(1H,d,J=9.0Hz),6.97(2H ,d,J=9.0Hz),7.19(2H,d,J=9.0Hz),8.00(1H,dd,J=8.9Hz,2.4Hz),...
Embodiment 2
[1857] Preparation of 1-(4-chlorobenzyl)-3-dimethylamino-6-(3-fluoro-4-isopropoxyphenylamino)benzene (I-123)
[1858] [chemical 122]
[1859]
[1860] To a mixture of 3-bromo-1-dimethylaminobenzene (0.3g, 1.5mmol) and THF (3mL), add 4-chlorobenzylzinc chloride (0.5MTHF solution, 6mL, 3mmol), triphenyl Phosphine (39.3mg, 0.15mmol) and palladium(II) acetate (17mg, 0.08mmol), stirred under reflux for 2 hours. Water (200 mL) was added to the reaction liquid, followed by extraction with ethyl acetate (200 mL). The extract was washed with saturated brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (ethyl acetate / hexane) to obtain 1-(4-chlorobenzyl)-3-dimethylaminobenzene (0.32 g, yield: 87 %).
[1861] 1H-NMR (δppmTMS / CDCl 3 ):2.89(6H,s),3.87(2H,s),6.48-6.59(4H,m),7.08-7.22(4H,m).
[1862]To a mixture of 1-(4-chlorobenzyl)-3-dimethylaminobenzene (120mg, 0.5m...
Embodiment 3
[1867] Preparation of 1-(4-chlorobenzyl)-3-(3-hydroxypropyloxy)-6-(3-fluoro-4-isopropoxyphenylamino)benzene (I-076)
[1868] [chem 123]
[1869]
[1870] To the mixed solution of 5-hydroxyl-2-nitrobenzaldehyde (3.0g, 18mmol) and DMF (10mL), add potassium carbonate (3.23g, 23.3mmol) and (3-bromopropoxyl (tert-butyl) Dimethylsilane (5.56g, 21.5mmol), stirred overnight at room temperature. Add water (200mL) to the reaction solution, and extract with ethyl acetate (200mL×2). The extract is washed with saturated brine (200mL), washed with Dry over anhydrous sodium sulfate, then concentrate under reduced pressure.The resulting residue is purified by silica gel column chromatography (ethyl acetate / hexane) to obtain 5-[3-(tert-butyldimethylsilane) in the form of light yellow oil Oxy)propyloxy]-2-nitrobenzaldehyde (3.0 g, yield: 49%).
[1871] 1H-NMR(δppmTMS / DMSO-d6):0.00(6H,s),0.83(9H,s),1.92(2H,q,J=5.7Hz),3.73(2H,t,J=5.7Hz),4.21 (2H,t,J=5.7Hz),7.22(1H,d,J=2.7Hz),7.33(1H,d,J=2.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com