Methods for preventing or treating metabolic syndrome
A technology for metabolic syndrome and fat metabolism, applied in metabolic diseases, drug combinations, pharmaceutical formulations, etc., and can solve the problem of unclear specific causes of metabolic syndrome.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] Example 1 - Treatment of Metabolic Syndrome in Rats Fed a High Fat Diet
[0249] The effect of aromatic-cationic peptides in treating subjects with metabolic syndrome was studied in the Sprague-Dawley rat model over a four-week period. This example describes the results of such experiments.
[0250] in vivo model. A rat model of metabolic syndrome was established by a 6-week combination of HFD and low-dose STZ (streptozotocin) (40 mg / kg) injections in SD rats. The same batch of rats (NRC) fed normal chow were used as controls.
[0251] research group. Arm A: NRC+PBS, subcutaneously, once daily (s.c.q.d.) (Monday to Friday) * , n=5; Group B: HFD+STZ+PBS, subcutaneous injection, once a day (Monday to Friday), n=8; Group C: SS-31 of HFD+STZ+10mg / kg, subcutaneous injection, every Once a day (Monday to Friday), n=8; Group D: HFD+STZ+10 mg / kg SS-20, subcutaneous injection, once a day (Monday to Friday), n=8. Table 7 shows the treatment regimen.
[0252] Table 7. Trea...
Embodiment 2-S
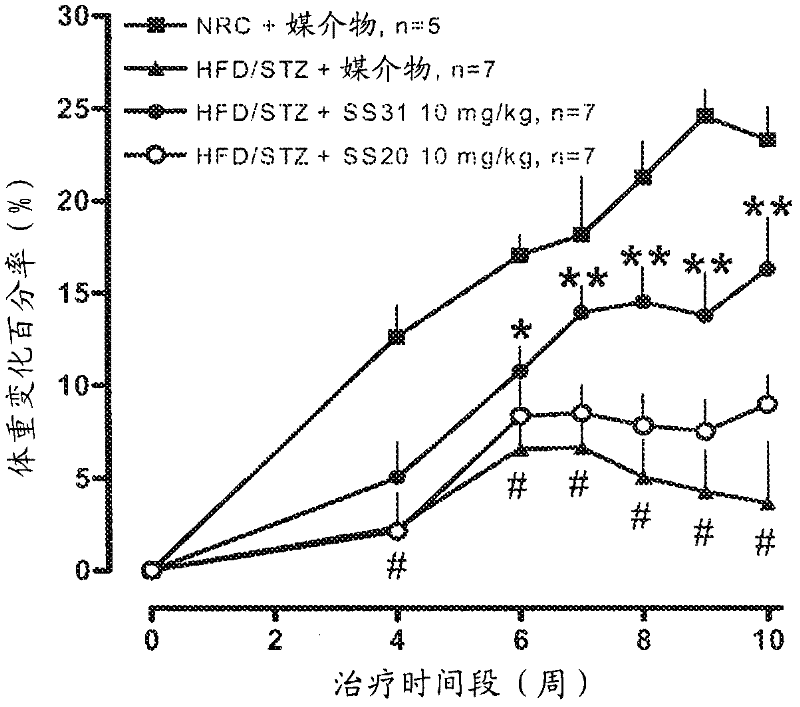
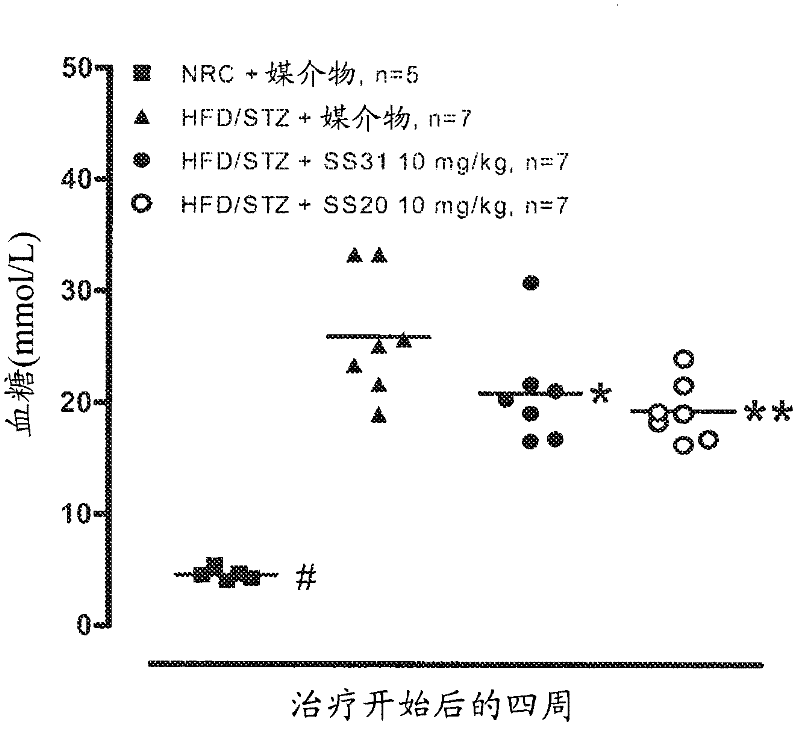
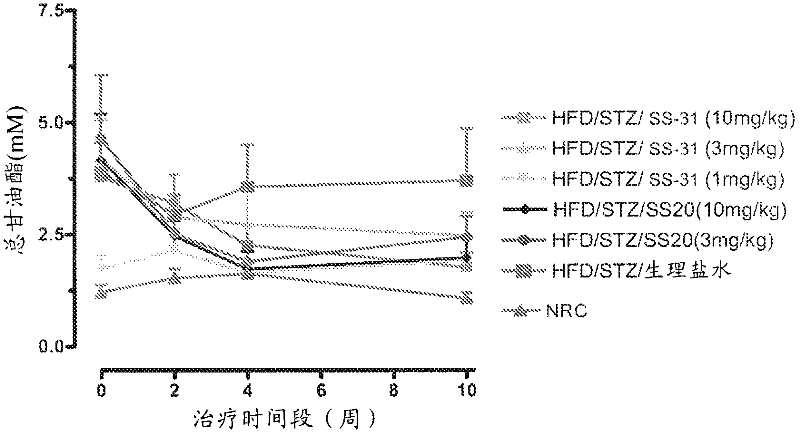
[0262] Example 2 - Effects of SS-20 and SS-31 on Metabolic Syndrome
[0263] This study was conducted to investigate the effect of SS-31 and SS-20 on a model of metabolic syndrome over a 10-week period. Rats were fed a high fat diet for 6 weeks followed by a single dose of STZ (30 mg / kg). The rats were maintained on HFD until 14 weeks after STZ administration. Control rats (NRC) fed normal mouse chow for 6 weeks were then dosed with STZ-free citrate buffer. After 5 months, use SS-31 (10mg / kg, 3mg / kg, 1mg / kg, subcutaneous injection, once a day), SS-20 (10mg / kg, 3mg / kg, subcutaneous injection, once a day) or Vehicle (saline) treated diabetic rats were treated 5 days a week for 10 weeks. The research groups are as follows:
[0264] Group A: HFD / STZ+10mg / kg of SS-31, subcutaneous injection, once a day (Monday to Friday), n=12;
[0265] Group B: HFD / STZ+3 mg / kg of SS-31, subcutaneous injection, once a day (Monday to Friday), n=12; Group C: HFD / STZ+1 mg / kg of SS-31, subcutane...
Embodiment 3
[0271] Example 3 - Effect of SS-20 and SS-31 on diet-induced obesity
[0272] To analyze the pharmacological effects of SS-31 and SS-20 on diet-induced obesity (DIO) in C57BL / 6 mice, test subjects were fed with normal chow or a high-fat diet with the components shown in Table 9.
[0273] Table 9. Food Components
[0274]
[0275] After several days of acclimatization, animals were weighed and randomly assigned to the following treatment groups.
[0276] Table 10. Treatment Groups
[0277] Group
[0278] Animals in groups B through I were switched to the HFD starting from the first day of treatment. During the entire treatment period, perform the following behaviors on specific days.
[0279] Table 11. Treatment options
[0280]
[0281] * On day 23, four animals were sacrificed in all groups. All remaining animals were sacrificed on the last day of the in vivo study (Day 109).
[0282] Dose Preparation & Administration. Treatments were performed once...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body mass index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com