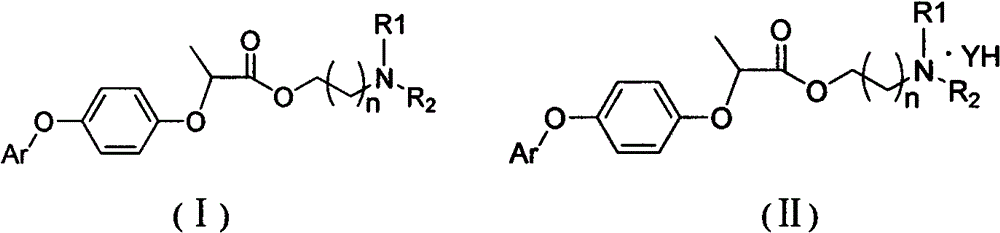
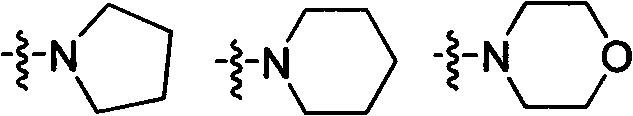
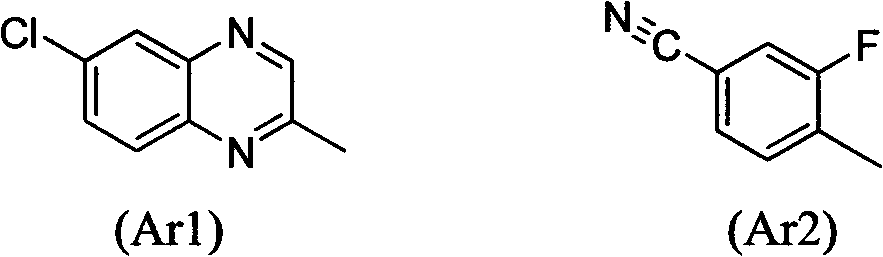Preparation and application of a new type of aryloxyphenoxycarboxylate derivatives soluble in water and oil
A technology of aryloxyphenoxycarboxylic acid and esters, which is applied to the preparation and herbicidal activity of novel aryloxyphenoxycarboxylic acid ester derivatives. It can solve the problems of preparation processing difficulties and achieve the effects of growth control, good herbicidal activity and broad spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0054] Synthesis of Compound 1:
[0055] 1.
[0056]
[0057] Add III (R type, 18.64g, 0.05mol, commercially available) and 40mlTHF into a 250ml reaction flask, stir until completely dissolved, add 2.2g of sodium hydroxide in 40ml of aqueous solution dropwise, stir at room temperature for 4 hours, stop stirring, and filter with suction Insoluble matter was filtered off, THF was removed under reduced pressure, and the aqueous solution was acidified with dilute hydrochloric acid to obtain 16.3 g of white solid (III-1).
[0058] 2.
[0059]
[0060] Add III-1 (1.72g, 0.005mol) and 15ml of dichloromethane into a 50ml reaction flask, add thionyl chloride (1.18g, 0.01mol) and 2 drops of DMF under stirring, heat to reflux for 5 hours, and precipitate to obtain a yellow color Oil (III-2) 1.70g.
[0061] 3.
[0062]
[0063] Add III-2 (1.70g, 0.0047mol) into a 50ml reaction flask, add 10ml of dichloromethane and stir to dissolve, add dropwise morpholine propanol (0.70g), t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com