Use of vip3ab in combination with cry1ca for management of resistant insects
A technology of insects and mixtures, applied in the field of combined use of Vip3Ab protein and Cry1Ca protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-V
[0092] Example 1 - Production and Trypsin Treatment of Vip3Ab and Cry1Ca Proteins
[0093] Genes encoding the Cry1Ca and Vip3Ab1 protoxins were expressed in a Pseudomonas fluorescens expression strain and the full-length proteins were isolated as insoluble inclusion bodies. Washed inclusion bodies were solubilized by stirring at 37°C for 2 hr in a buffer containing 20 mM CAPS buffer, pH 11, +10 mM DDT, +0.1% 2-mercaptoethanol. The solution was centrifuged at 27,000 xg for 10 min at 37°C, and the supernatant was treated with 0.5% (w / v) TCPK-treated trypsin (Sigma). The solution was incubated with mixing for an additional 1 h at room temperature, filtered and then loaded onto a Pharmacia Mono Q 1010 column equilibrated with 20 mM CAPS pH 10.5. After washing the loaded column with 2 column volumes of buffer, the truncated toxin was eluted with a linear gradient of 0-0.5 M NaCl in 20 mM CAPS at a flow rate of 1.0 ml / min over 15 column volumes. Purified tryptic truncated Cry pr...
Embodiment 2-C
[0095] Example 2-Iodination of Cry1Ca core toxin protein
[0096] Although in a few selected examples Cry1Ca can be radiolabeled and functions well in receptor binding assays, previous work has shown that Cry1Ca is very difficult to radiolabel with traditional methods. we decided to use 125 Cry1Ca was radiolabeled with radiolabeled fluorescein-5-maleimide, a method already used for active radiolabeling of Cry1Fa (Prov. 69919). Iodination of fluorescein-5-maleimide and subsequent conjugation of this radiolabeled chemical to CrylCa results in specific radiolabeling of cysteines of the protein. Thus, this labeling procedure is highly specific at the residues labeled. The CrylCa core toxin segment (residues 29-619) contains two cysteine amino acid residues, at positions 210 and 438. Palmer et al. (1997) demonstrated that the phenyl ring of fluorescein-5-maleimide can be radioiodinated and then reacted with proteins containing sulfhydryl groups (e.g. as donated by free cyste...
Embodiment 3
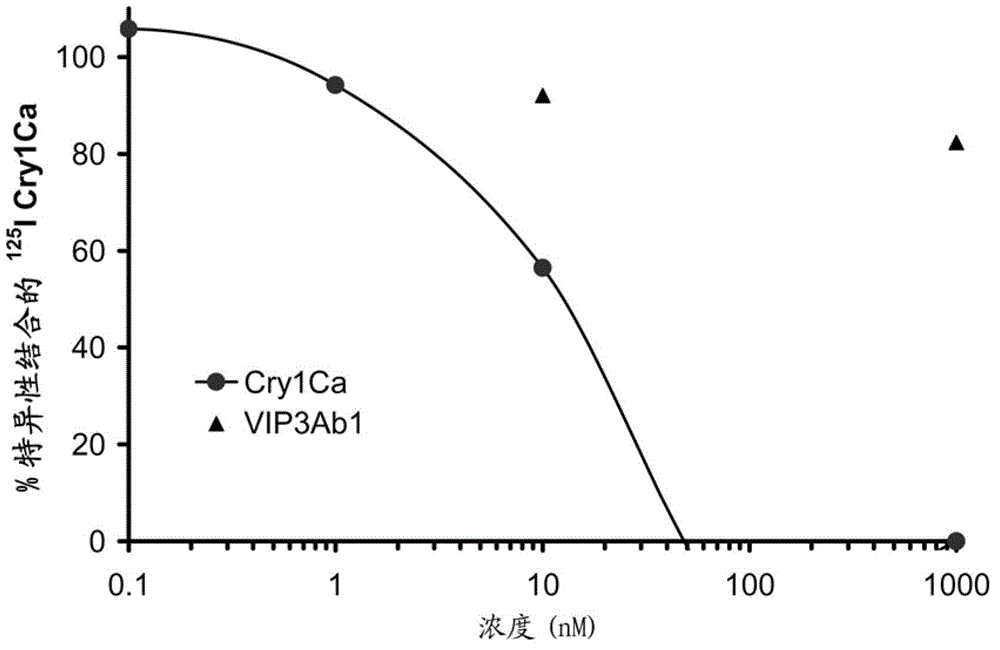
[0099] Example 3-Cry1Ca and Vip3Ab core toxin protein from Fall Armyworm (S.frugiperda) Competitive binding assay of BBMVs
[0100] Homologous and heterologous competition binding assays were performed with 150 μg / mL BBMV protein and 2 nM of 125I-radiolabeled Cry1Ca core toxin protein. Concentrations of 0.1, 1, 10, 100 and 1000 nM of cognate competing non-radiolabeled Cry1Ca core toxin protein added to the reaction mixture. Heterologous trypsin-truncated Vip3Ab protein was tested at 10 and 1,000 nM, which was added simultaneously with radioactive CrylCa core toxin protein to ensure true binding competition. Incubation was performed at 28° for 1 hr. The amount of 125I-labeled CrylCa core toxin protein not bound to BBMV's (i.e., not bound to insect receptor protein) was compared to bound protein by centrifuging the BBMV mixture at 16,000 x g for 8 min and removing the supernatant from the resulting pellet. separate. Precipitate with ice-cold binding buffer (PBS; 11.9 mM N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com