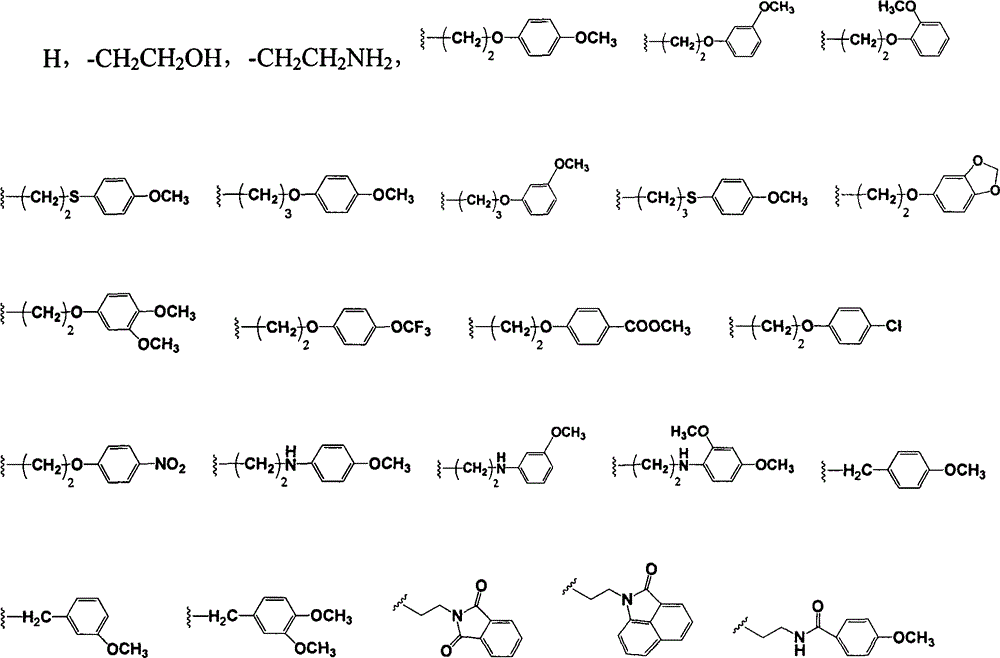
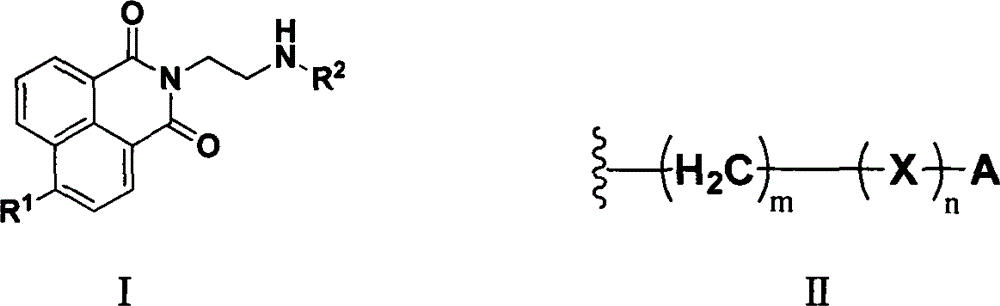Naphthylamine derivative and purpose thereof
A technology of naphthalimide and derivatives, which is applied in the field of naphthalimide derivatives, can solve the problems of not being able to be used for treatment and high clinical toxicity, and achieve the effect of good inhibitory activity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 2-(2-aminoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Al)
[0042]
[0043] In a 25mL round bottom flask, add 1,8-naphthalene anhydride (2g, 10.1mmol), 50mL of absolute ethanol and ethylenediamine (1.35mL, 20.2mmol) successively, and the mixture is heated to reflux for 2 hours. After the reaction is completed, depressurize The solvent and excess ethylenediamine were evaporated, and the crude product was recrystallized from absolute ethanol to obtain product A1 (1.8 g) as a light yellow solid with a yield of 74%.
[0044] Melting point: 141-142°C.
[0045] 1 H NMR (400MHz, DMSO-d 6 ): δ=8.34(d, J=7.6Hz, 2H), 8.31(d, J=8.0Hz, 2H), 7.75(dd, J=8.0, 7.6Hz, 2H), 4.01(t, J=6.8Hz , 2H), 2.79(t, J=6.8Hz, 2H), 2.62ppm(br, 2H); 13 CNMR (100MHz, DMSO-d 6 ): δ=163.9, 134.4, 131.5, 130.9, 127.7, 127.4, 122.4, 43.2, 40.2ppm; HRMS-ESI (m / z): calculated value: C 14 h 13 N 2 o 2 [M+H] + , 241.0977; experimental value, 241.0978.
Embodiment 2
[0047] Synthesis of 2-(2-((2-hydroxyethyl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (A2)
[0048]
[0049] In a 25mL round bottom flask, add 1,8-naphthalene anhydride (198mg, 1.00mmol) successively, 10mL absolute ethanol and hydroxyethylethylenediamine (140mg, 1.00mmol), the mixture was heated to reflux for 2 hours, after the reaction was complete, The solvent was evaporated under reduced pressure, and the crude product was recrystallized from absolute ethanol to obtain the product A2 (241 mg) as a white solid with a yield of 85%.
[0050] 1 H NMR (CDCl 3 , 400MHz): δ=8.55(d, J=7.2Hz, 2H), 8.17(d, J=8.0Hz, 2H), 7.71(dd, J=8.0, 7.2Hz, 2H), 4.32(t, J= 6.0Hz, 2H), 3.61(t, J=4.4Hz, 2H), 3.02(t, J=6.0Hz, 2H), 2.84(t, J=4.4Hz, 2H), 2.2ppm(br, 2H); 13 C NMR (100MHz, CDCl 3 ): δ=164.5, 134.0, 131.5, 131.3, 128.1, 126.9, 122.5, 61.0, 50.9, 47.4, 40.1 ppm. EI MS (m / e): 285.4 (M+1, 100).
Embodiment 3
[0052] Synthesis of 2-(2-((2-aminoethyl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (A3)
[0053]
[0054] Add 1,8-naphthalene anhydride (198mg, 1.0mmol), 20mL absolute ethanol and 2mL diethylenetriamine (excessive amount) successively in a 50mL round bottom flask, and heat the mixture to reflux for 2 hours. After the reaction is complete, filter and collect the filtrate , evaporated the solvent under reduced pressure, washed with water, extracted three times with dichloromethane, collected the organic layer, evaporated the solvent under reduced pressure to obtain a white solid, and the crude product was recrystallized with absolute ethanol to obtain the white solid product A3 (189mg), the yield 67 %.
[0055] 1 H NMR (CDCl 3 , 400MHz): δ=8.54(d, J=6.8Hz, 2H), 8.17(d, J=8.0Hz, 4H), 7.71(t, J=7.6Hz, 2H), 4.32(t, J=6.0Hz , 2H), 3.01(t, J=6.4Hz, 2H), 2.77ppm(s, 4H); 13 C NMR (100MHz, CDCl 3 ): δ=164.0, 133.6, 131.2, 130.9, 127.8, 126.6, 122.2, 51.8, 47.2, 41.4, 39...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap