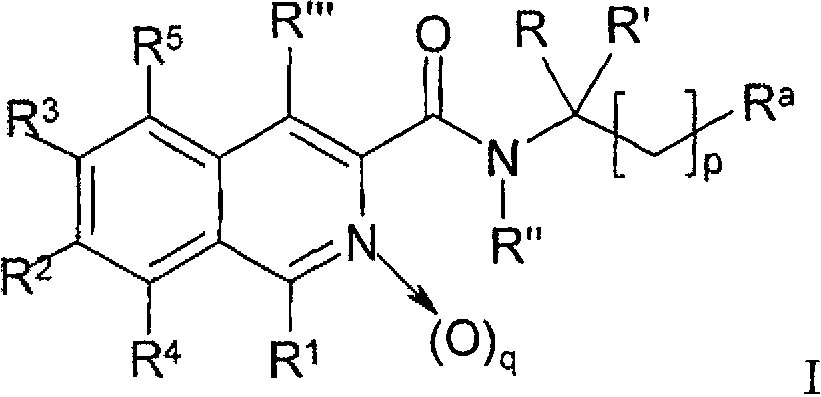
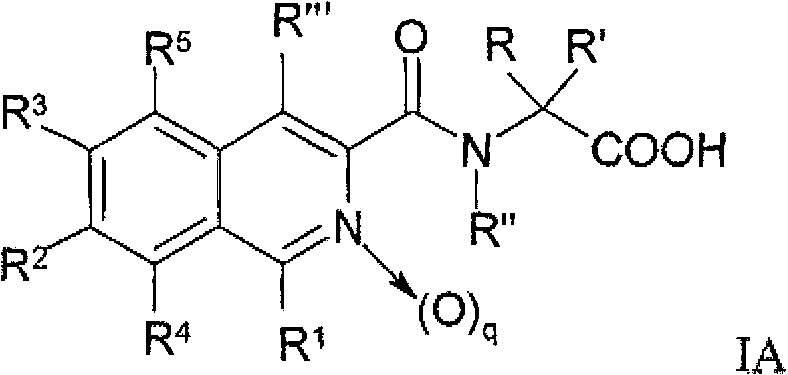
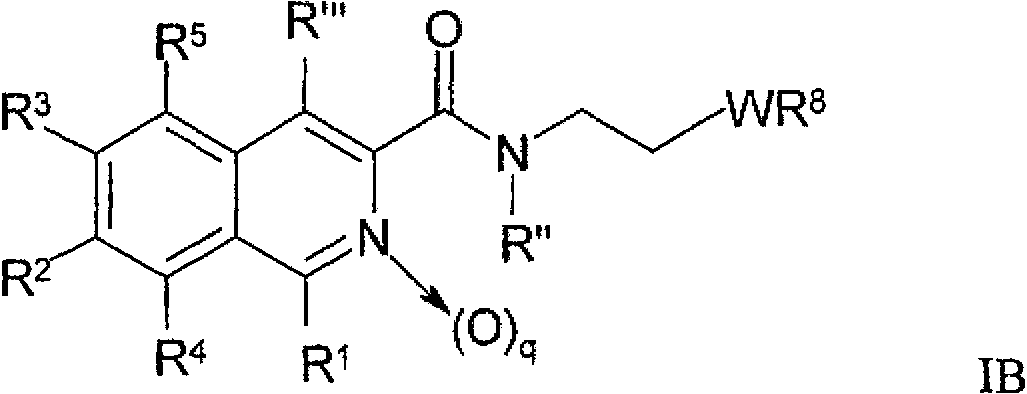
Nitrogen-containing heteroaryl compounds and their use in increasing endogeneous erythropoietin
A technology of heteroaryl compounds, applied in the field of nitrogen-containing heteroaryl compounds and their use in increasing endogenous erythropoietin, capable of solving problems such as tissue damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0684] The invention may be further understood by reference to the following examples, which are intended purely to demonstrate the invention. The present invention is not limited to the scope of the exemplary embodiment, which is only used as an illustration of a single aspect of the present invention. Any functionally equivalent methods are intended to be within the scope of this invention. Various modifications of the invention in addition to the modifications described herein will become apparent to those skilled in the art from the foregoing description and accompanying drawings. Such modifications are within the scope of the appended claims.
[0685] All temperatures are in degrees Celsius unless otherwise stated. Also, abbreviations appearing in these examples and elsewhere have the following meanings:
[0686] μl = microliter
[0687] amu = atomic mass unit
[0688] atm = atmospheric pressure
[0689] bs = broad singlet
[0690] ClCO 2 iBu = isobutyl chloroform...
example A-1
[0741] (S)-2-[(6-Benzyloxy-1-chloro-4-hydroxy-isoquinoline-3-carbonyl)-amino]-propionic acid
[0742] a.(S)-2-[(6-Benzyloxy-1-chloro-4-hydroxy-isoquinoline-3-carbonyl)-amino]-propionic acid methyl ester
[0743] In 15ml CH at room temperature 2 Cl 2 0.33 g of 6-benzyloxy-1-chloro-4-hydroxy-isoquinoline-3-carboxylic acid (available according to Weidmann et al. US Patent 6,093,730, 10 / 1998), 0.5 ml of triethylamine, 0.38 g HATU and 0.151 g of commercially available L-alanine methyl ester hydrochloride for 18 h yielded (S)-2-[(6 -Benzyloxy-1-chloro-4-hydroxy-isoquinoline-3-carbonyl)-amino]-propionic acid methyl ester 0.220g, MS-(+)-ion, M+1=415.8amu.
[0744] b. (S)-2-[(6-Benzyloxy-1-chloro-4-hydroxy-isoquinoline-3-carbonyl)-amino]-propionic acid
[0745]0.200 g of the (S) methyl ester described in Example A-1a) and 15 ml of a 1.5M NaOH solution in methanol were stirred at room temperature for 3 h and concentrated. The residue was dissolved in water and extracted with EtOAc....
example A-2
[0747] (R)-2-[(1-Chloro-4-hydroxy-isoquinoline-3-carbonyl)-amino]-3-hydroxy-propionic acid
[0748] a. (1,3-Dioxy-1,3-dihydro-isoindol-2-yl)-butyl acetate
[0749] A mixture of 160ml butanol, 20.0g (1,3-dioxo-1,3-dihydro-isoindol-2-yl)-acetic acid (94.6mmol) and 2.0ml concentrated sulfuric acid was refluxed for 24h under stirring. Then 5 g of sodium bicarbonate were added in portions, stirring was continued for 5 min at room temperature and the solvent was evaporated in vacuo. The residue was partitioned between 100 mL of water and 100 mL of ethyl acetate. The organic phase was washed with 100 ml brine, dried over sodium sulfate and evaporated in vacuo to give a yellowish oil which then solidified. 24.02 g of the title compound were obtained; MS-(+)-ion:
[0750] b. Butyl 1,4-dihydroxy-isoquinoline-3-carboxylate
[0751] 4.41 g of sodium (190 mmol) were dissolved in 250 ml of n-butanol with stirring. After complete dissolution of the sodium, the solution was cooled to am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com