Preparation method of new polypeptide, and application of new polypeptide in treatment
A technology of pharmaceutical preparations and amino acids, applied in the field of medicine and biology, can solve problems such as side effects and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
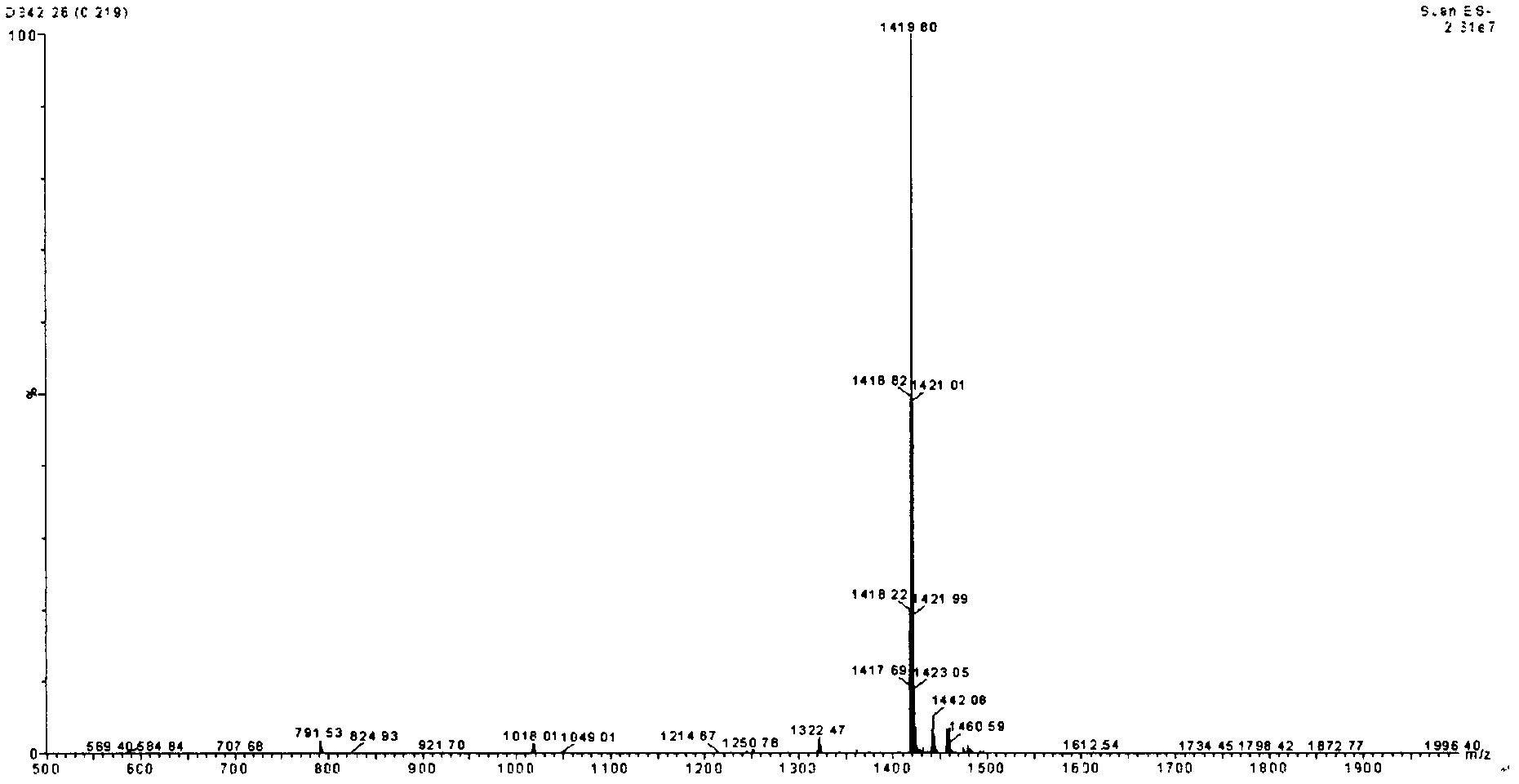
[0019] Example 1: Chemical synthesis and purification of polypeptides:
[0020] Taking the amino acid sequence shown in SEQ ID No as an example, the polypeptide is chemically synthesized.
[0021] Synthesized by Fmoc solid-phase method:
[0022] Adopt solid-phase synthesis instrument, select the val-2 chlorotrityl resin of 0.5 gram as initial resin, add successively in the synthesis reaction tube:
[0023] Fmoc-arg(pbf)-OH, Fmoc-Gln(trt)-OH, Fmoc-lys(boc)-OH, Fmoc-cys(trt)-OH, Fmoc-phe-OH, Fmoc-arg(pbf)-OH , Fmoc-arg(pbf)-OH, Fmoc-arg(pbf)-OH, Fmoc-cys(trt)-OH, Fmoc-asn(trt)-OH, Fmoc-tyr(tbu)-OH, Fmoc-Ile- Oh
[0024] Add DIC / DMF solution, HOBT / DMF solution and DMF solution to the reagent bottle. Start the synthesis instrument and connect it to the computer, enter the synthesis program, and start the synthesis.
[0025] Compositing post-processing:
[0026] Take the resin out from the synthesis reaction column, put it into a glass container with a sintered glass filter a...
Embodiment 2
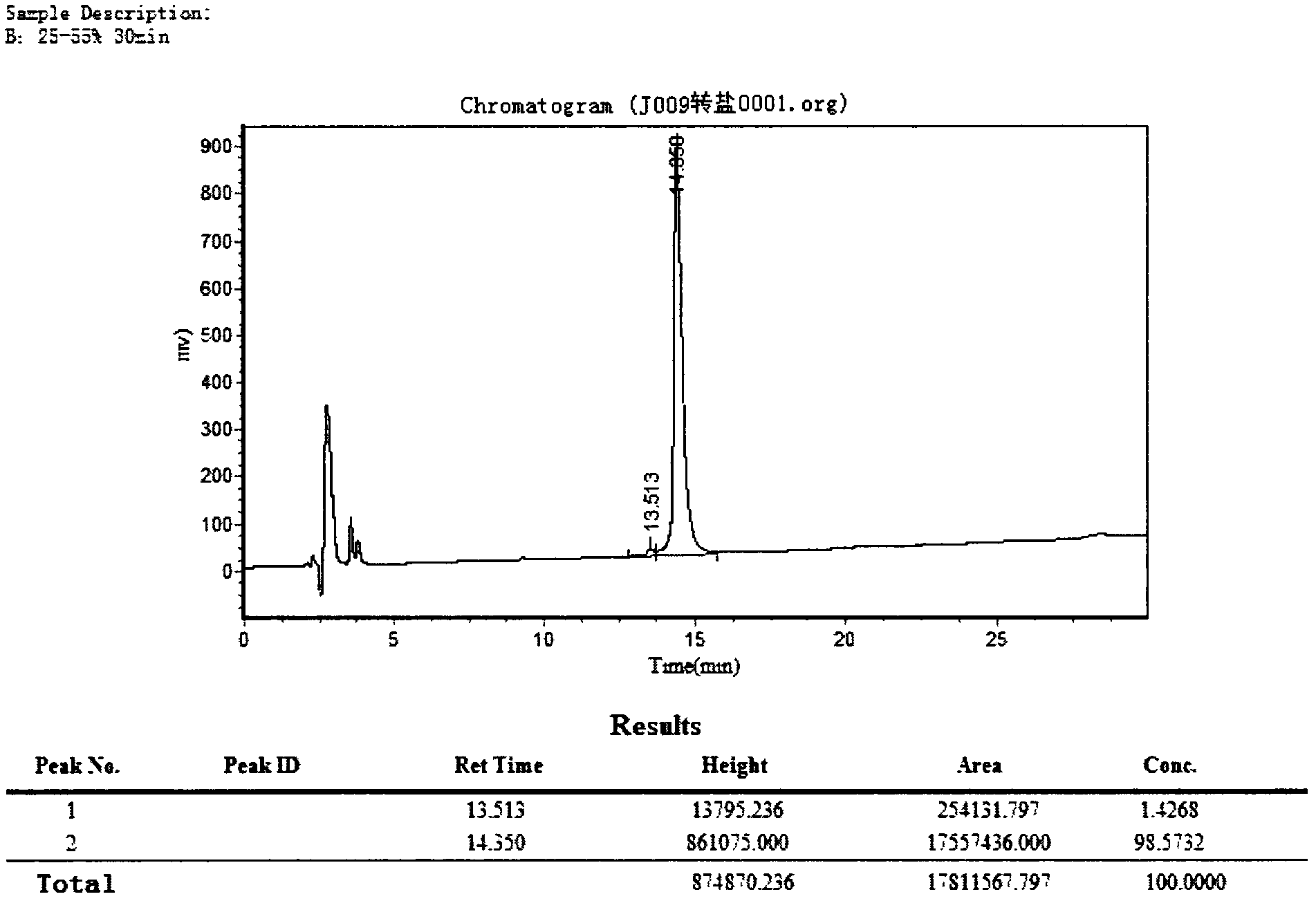
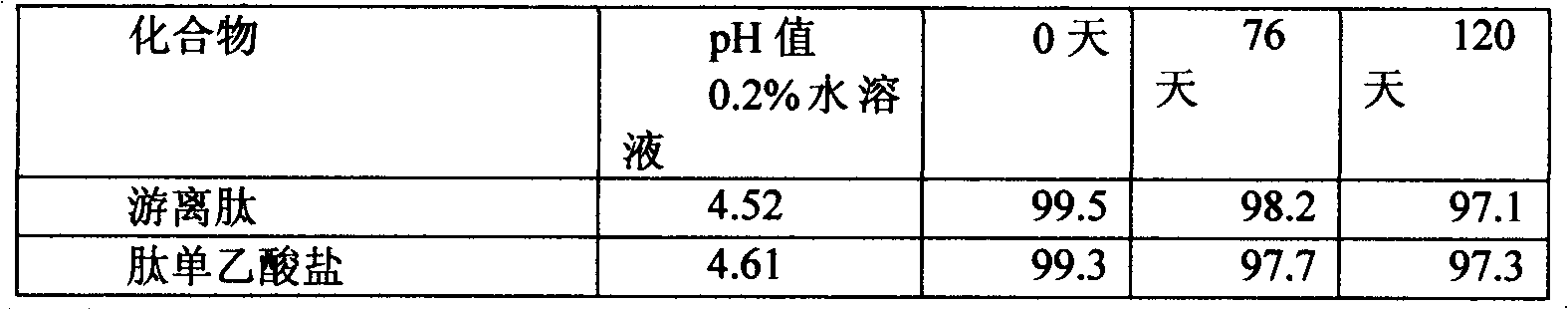
[0042] Embodiment 2: stability test
[0043] The polypeptide of the present invention was incubated at 40°C for 76 days and 120 days to test its stability. The stability test was determined by HPLC method. The concentration of polypeptide in aqueous solution is 0.2% (w / v): column, Kromasil100, 5u, 250x 4.6mm; mobile phase, water / acetonitrile solution of 0.1% trifluoroacetic acid (0 to 50vol.%), within 25 minutes Gradient elution, flow rate 1ml / min; detection: UV, 218nm.
[0044] For comparison, the free polypeptide and its monoacetate salt were used.
[0045] Table 1
[0046]
[0047] It can be shown from the data in Table 1 that the stability of the polypeptide of the present invention is very high. The solution still has more than 97% of the target substance after 120 days at 40°C.
Embodiment 3
[0048] Embodiment 3: the toxicity of polypeptide of the present invention
[0049] The acute toxicity of the polypeptide of the present invention is determined by intragastric administration and injection in mice.
[0050] Solution preparation:
[0051] Use 25 mg of the polypeptide of the present invention, add sterilized water to 20 ml, and prepare a solution with a concentration of 1.25 mg / ml for oral gavage.
[0052] Use 25 mg of the polypeptide of the present invention, add sterilized water to 10 ml, and prepare a solution with a concentration of 2.5 mg / ml for intraperitoneal injection.
[0053] Dosage Calculation:
[0054] The volume of oral gavage administration to mice is 40 ml / kg body weight, so the administration dose is: 1.25 mg / ml*40 ml / kg=50 mg / kg.
[0055]The volume of intraperitoneal injection in mice is 20ml / kg body weight, so the dosage is: 2.5mg / ml*20ml / kg=50mg / kg.
[0056] Take 80 healthy mice and divide them into four groups for experiment. After admini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap