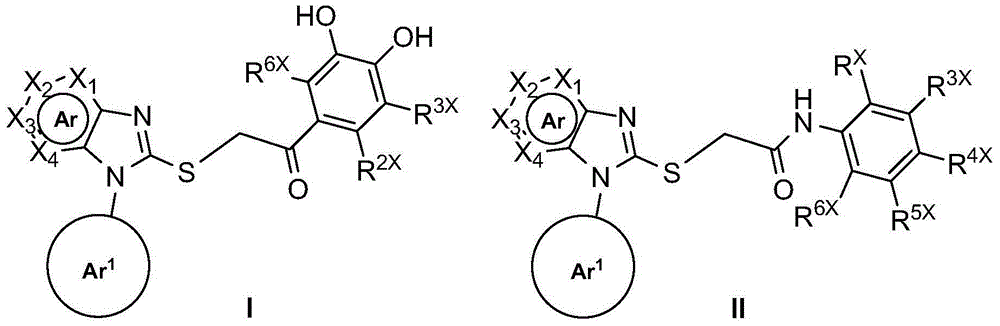
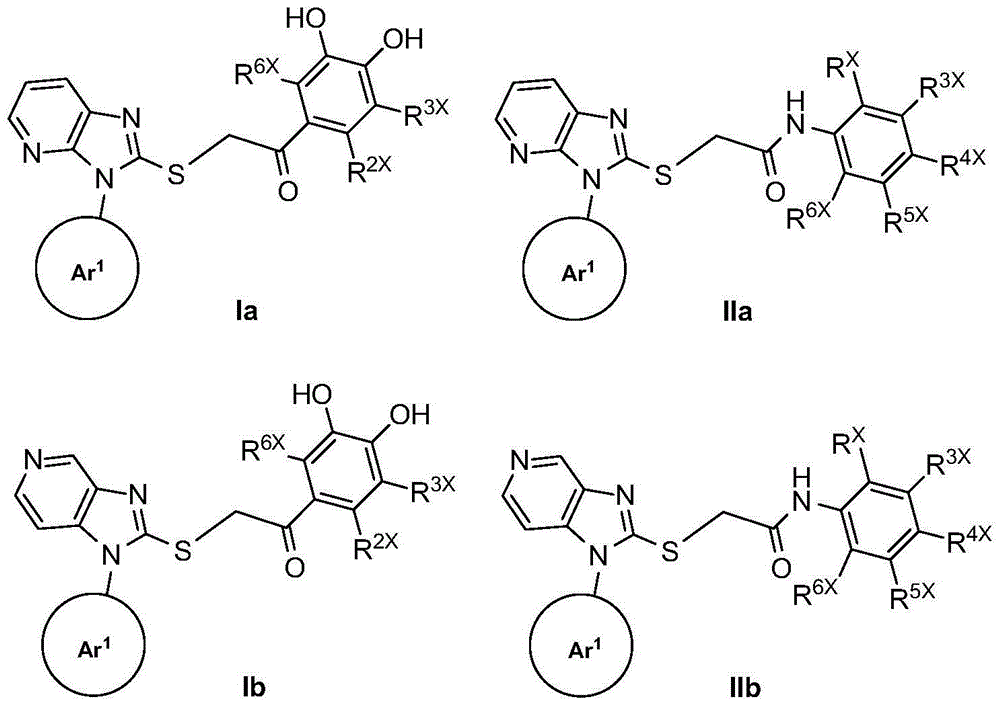
Substituted mercapto six-membered aromatic heterocyclic imidazole derivatives and its preparation method and application
A technology of derivatives and aromatic heterocycles, applied in the field of medicine, can solve problems such as endangering human life and health, and unsatisfactory treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
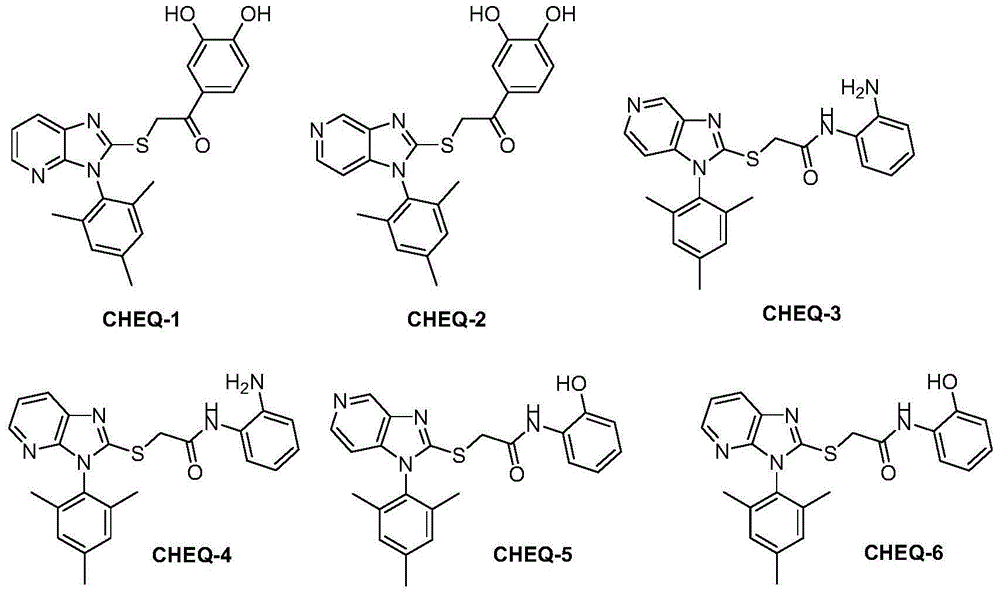
[0080] Example 1: 1-(3,4-dihydroxyphenyl)-2-(3-(2,4,6-trimethylbenzene)-3H-imidazo[4,5-b]pyridine-2-mercapto ) ethyl ketone (CHEQ-1, Ar 1 =2,4,6-Trimethylphenyl) Preparation
[0081] 20 mL of 1,2,-dichloroethane solution was cooled to 5-10°C, aluminum trichloride (6.0 g, 45 mmol) was added slowly, and the mixture was stirred at 5-10°C for 30 minutes. Then slowly add catechol (a1, R 2X =R 3X =R 6X =H) (2.0 g, 18.1 mmol), the addition was complete and the reaction was stirred at this temperature for 20 minutes. Chloroacetyl chloride (2.2 g, 19.3 mmol) was then added dropwise, keeping the temperature at 5-10°C. After the addition, the temperature was raised to room temperature and stirred for about 20 hours. TLC detected that the reaction was complete, slowly added 40 mL of dilute hydrochloric acid solution at 5-10°C, and stirred at room temperature for 2-3 hours. The solid precipitated, was suction filtered, washed with water, the undried solid was suspended in dilute ace...
Embodiment 2
[0083] Example 2: 1-(3,4-dihydroxyphenyl)-2-(1-(2,4,6-trimethylbenzene)-1H-imidazo[4,5-c]pyridine-2-mercapto ) ethyl ketone (CHEQ-2, Ar 1 =2,4,6-Trimethylphenyl) Preparation
[0084] The operation method is the same as the preparation of CHEQ1 in Example 1, except that 1-(2,4,6-trimethylphenyl)-1H-imidazol[4,5-c]pyridine-2-mercapto (PDMZ2SH, Ar 1 =2,4,6-trimethylphenyl). The obtained product is off-white powder, 0.28g, yield: 67%; melting point: 256-258°C. 1 H NMR (400MHz, DMSO-d6, ppm) δ: 9.78 (2H, brs), 8.88 (1H, s, C 4 -imidazo[4,5-c]pyridine-H), 8.26 (1H,d,J=4.8Hz,C 6 -imidazo[4,5-c]pyridine-H),7.49(1H,dd,J=1.5,8.2Hz,C 6 -Ph'-H),7.41(1H,s,C 2 -Ph'-H),7.19(2H,s,C 3,5 -Ph-H),6.97(1H,d,J=4.8Hz,C 7 -imidazo[4,5-c]pyridine-H),6.86(1H,d,J=8.2Hz,C 5 -Ph'-H),5.03(2H,s,S-CH 2 ),2.38(3H,s,Me),1.88(6H,s,2×Me).MS(ESI):m / z420.3(M+1).C 23 h 21 N 3 o 3 S(419.13).
Embodiment 3
[0085] Example 3: N-(2-aminophenyl)-2-(1-(2,4,6-trimethylbenzene)-1H-imidazo[4,5-c]pyridine-2-mercapto)acetamide (CHEQ-3, Ar 1 =2,4,6-trimethylphenyl,R 3X =R 4X =R 5X =R 6X =H) Preparation
[0086] 2-nitroaniline (b3, R 3X =R 4X =R 5X =R 6X =H) (2.0 g, 14.5 mmol) was dissolved in dichloromethane (15 mL), then triethylamine (2.5 mL, 17.4 mmol) was added and stirred for 10 minutes. Then slowly add 10 mL of dichloromethane solution of chloroacetyl chloride (1.38 mL, 17.4 mmol) dropwise into the above solution, stir at room temperature for 3 hours, add 10 mL of ice water, wash the organic phase with water (30 mL), and extract the aqueous phase with dichloromethane (3×15mL), the organic phases were combined, dried over anhydrous sodium sulfate, suction filtered, and concentrated under reduced pressure to obtain 2-chloro-N-(2-nitrophenyl)acetamide (b4, R 3X =R 4X =R 5X =R 6X =H), yellow crystals, yield: 83.6%, the purity is enough to carry out the next reaction directly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com