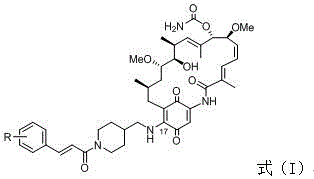
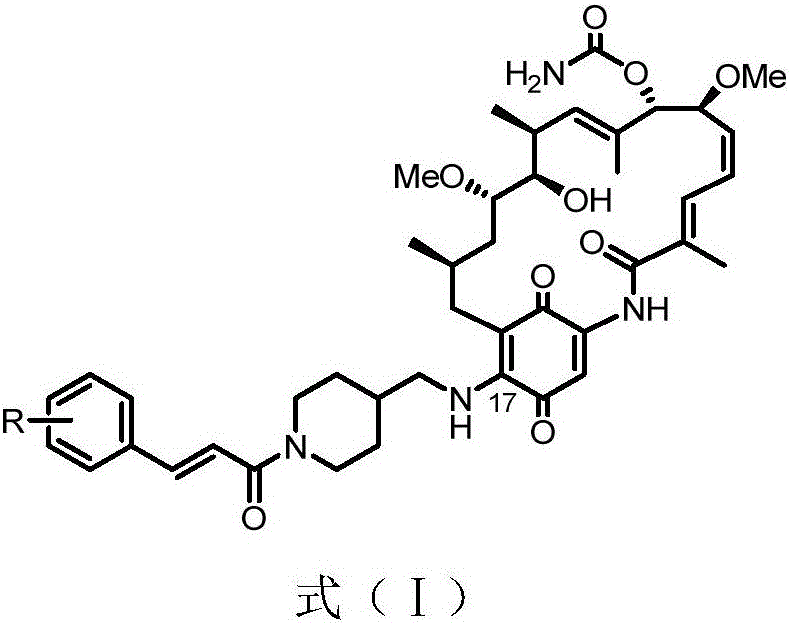
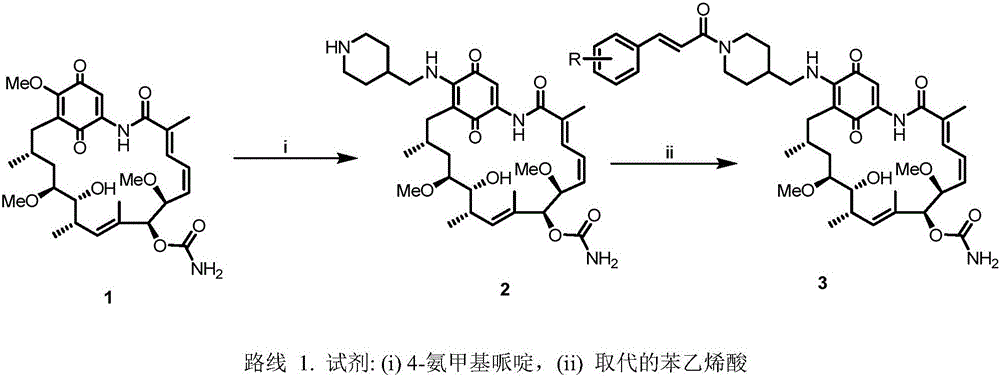
A class of geldanamycin derivatives capable of inhibiting castration-resistant prostate cancer and osteogenic metastasis and applications thereof
A geldanamycin, anti-prostate cancer technology, applied in the direction of organic active ingredients, anti-tumor drugs, drug combinations, etc., can solve the problems of poor water solubility, increased anti-tumor activity, strong liver toxicity, etc. Good medicinal prospects, strong pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the synthesis of 17-((1-((E)-3-(4-hydroxycinnamoyl) piperidin-4-yl) methylamino)-17-desmethoxygeldanamycin ( 3a)
[0034] Weigh GA (1) (28.0 mg, 0.05 mmol, 1 equiv.) in a 50 mL round bottom flask and dissolve it in chloroform (5 mL), then add 4-aminomethylpiperidine (57.1 mg, 0.50 mmol, 10 equiv.) After the reaction solution was stirred in the dark for 6 hours, TLC monitored the reaction process. After the reaction was completed, it was washed 3 times with water (25mL x 3), washed 3 times with saturated brine (25mL x 3), dried over anhydrous sodium sulfate, and concentrated to obtain the product 2, directly used in the next step without purification. Intermediate 2 obtained above was dissolved in DMF (8 mL), NHS (6.3 mg, 0.055 mmol, 1.1 equiv.), EDC (10.4 mg, 0.055 mmol, 1.1 equiv.) and 4-hydroxycinnamic acid (8.2 mg , 0.050 mmol, 1.1 equiv.). The reaction solution was stirred at room temperature in the dark, and TLC monitored the reaction progress. Aft...
Embodiment 2
[0037] Example 2: In vitro anti-tumor cell activity screening test of geldanamycin derivatives
[0038] testing method:
[0039] The antitumor activity of various compounds was evaluated by MTT method. Cells in the logarithmic growth phase were digested with trypsin to make a single cell suspension. Count with a hemocytometer and dilute to a cell concentration of 6 x 10 4 cells / mL, seeded in 96-well cell culture plate, 80 μL per well. Another 3 wells with no cells and only the same volume of medium were used as blank control. Continue to cultivate for 24h, and then add 20 μL of the sample diluted with cell culture medium. At the same time, add 20 μL of diluted cisplatin to the positive control wells, and add DMSO and 20 μL of culture medium at the same dilution to the negative control wells and blank control wells, respectively. Continue culturing for 72 hours, then add 10 μL of 5 mg / mL MTT solution to each well, incubate at 37° C. for 3 hours, then add 100 μL MTT stop so...
Embodiment 3
[0051] Example 3: Detection of apoptosis induced by geldanamycin derivative 3b in androgen-independent prostate cancer cell line Du-145
[0052] testing method:
[0053] The apoptosis-inducing effect of 3b on Du-145 cells was detected by Annexin V-FITC / PI double staining method. The cells in the logarithmic growth phase were trypsinized to make a single cell suspension and seeded in a 6-well cell culture plate with 3×105 cells per well. Continue culturing for 24 hours, replace with fresh medium, and add different concentrations of sample 3b diluted with cell culture medium. Continue culturing for 24 hours, then use EDTA-free trypsin to collect cells (including suspended cells in culture medium), centrifuge at 1000r / min for 5min, remove the supernatant, and wash the cells twice with cold PBS (1000r / min, centrifuge 5min), suspend the cells with 400ul 1X BindingBuffer, the concentration is about 1×106 cells / ml, then add 5ul AnnexinV-FITC to the cell suspension, mix gently and i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com