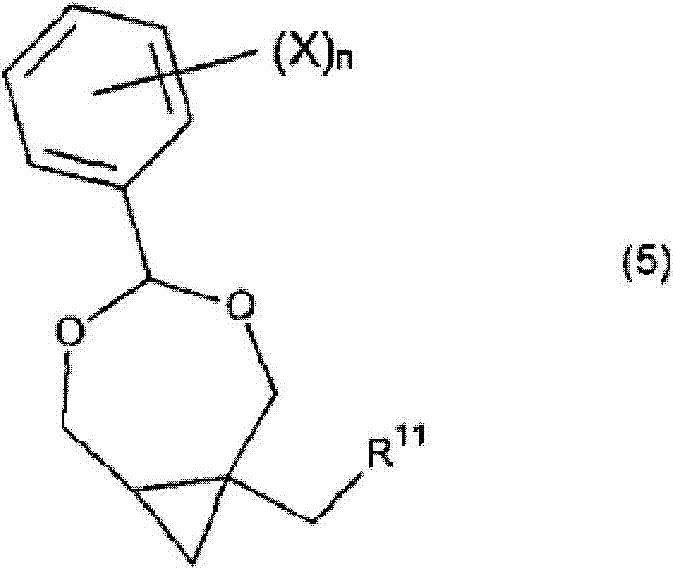
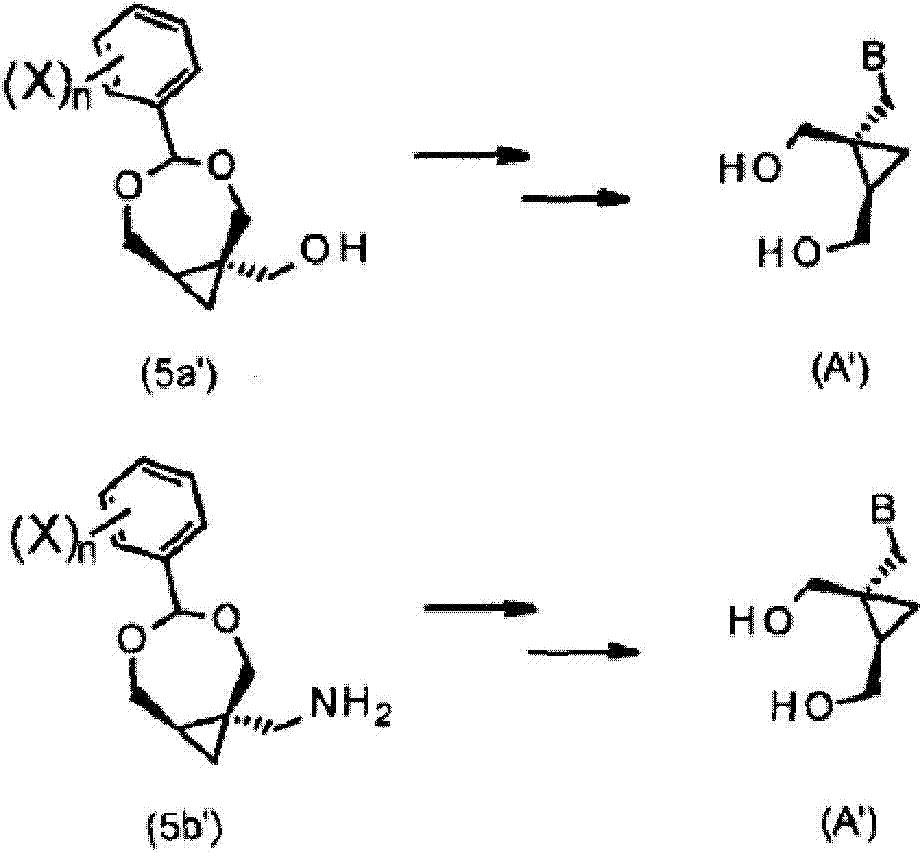
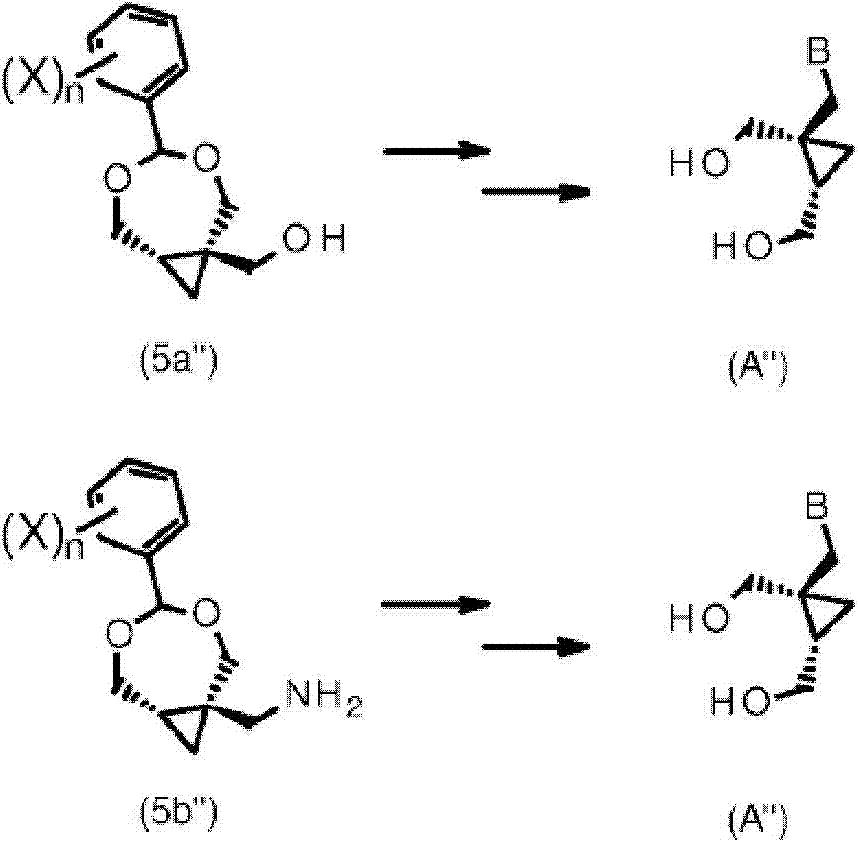
Process for the preparation of cyclopropane derivatives
一种烷基、化合物的技术,应用在环丙烷衍生物的制备领域,能够解决费时保护和去保护步骤、低稳定性中间体、危险产物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0226] The following examples are provided for the purpose of illustrating the invention but are in no way intended and should in no way be construed as limiting the scope of the invention.
[0227] 1) Preparation of (1R, 2R)-1,2-two (hydroxymethyl) ethyl cyclopropanecarboxylate (2c)
[0228]
[0229] In the reactor, ethanol (82.4 L) was added followed by ethyl (1S,5R)-2-oxo-3-oxabicyclo[3.1.0]hexane-1-carboxylate (10.3kg, 64.6mol) and cooled to 10°C. Sodium borohydride (1.85 kg, 51.7 mol) was added in portions over a period of 1 hour by maintaining the reaction temperature between 10-15°C. The reaction temperature was raised to 20-25°C and maintained for 1 hour. The progress of the reaction was monitored by HPLC. After the reaction was complete, the reaction mass was cooled to 0-5°C. The pH of the reaction mass was adjusted to 7.0 using 1.5N aqueous HCl at 0-5°C during 1 hour. Ethyl acetate (34.0 L) was added to the reaction mass and the solid was filtered. The clear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com