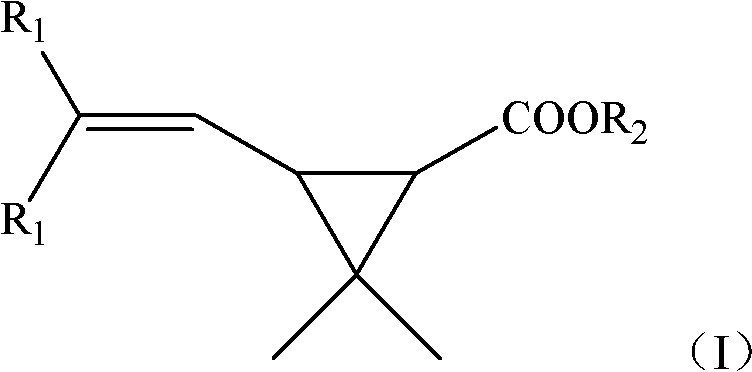
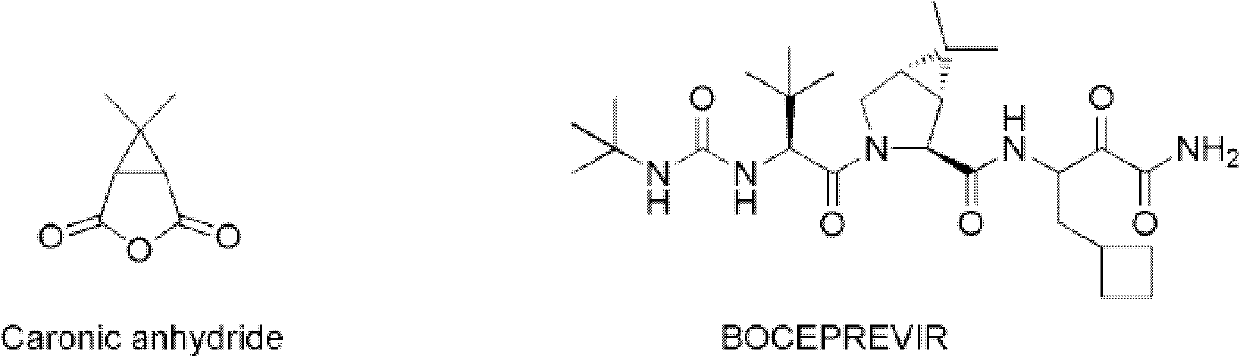
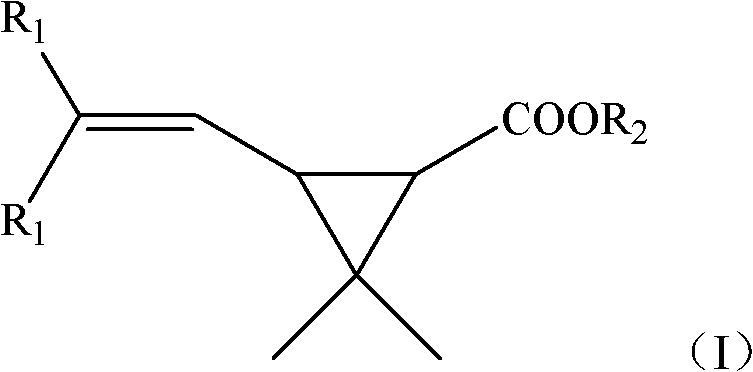
New synthetic method of Caronic anhydride
A synthetic method, the technology of chrysanthemic acid ester, which is applied in the new field of synthesizing carronic acid anhydride, can solve the problems of uneconomical, unenvironmental protection, unsuitable for large-scale industrial production, etc., and achieve the effect of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: in 500mL flask, add 200g ethyl acetate, drop into permethrin ethyl 94.8g (0.4mol) again, cool down to-5 ℃, begin to pass into the ozone of volume concentration 3% with the flow rate of 2L / min ( The remainder is air, the same below) to react, during the reaction process, carry out central control analysis of raw materials by gas spectrometry, and stop passing ozone after the conversion rate of ethyl permethrin is greater than 99%. After the reaction, add water, wash and separate the oil layer with water, wash twice, each time with 100ml of water, and after the washing is completed, the oil layer is concentrated by rotary evaporation to remove the solvent, and then 70g of 30% liquid caustic soda is added dropwise to the remaining reaction-finished material to carry out Saponification reaction, at this time, the pH is greater than 12, and the temperature is kept at 50-60°C for 2 hours, and then 30% hydrochloric acid is added dropwise to the reaction liquid for acidi...
Embodiment 2
[0031] Step 1: Add 80g of 50% mass concentration of acetic acid aqueous solution into a 500mL flask, then add 80g (0.4mol) of DE chrysanthemum acid ethyl ester, cool down to 10°C, and start to feed ozone with a volume concentration of 1.5% at a flow rate of 3L / min Carry out the reaction, and carry out central control analysis through gas spectrum during the reaction process, and stop passing ozone after the conversion rate of ethyl chrysanthemum acid is greater than 99%. After the reaction is completed, the layers are separated, and then 90g of 30% liquid caustic soda is added dropwise to the above-mentioned reaction finished material to carry out saponification reaction. % hydrochloric acid acidification reaction until the pH value reaches 1-2, the reaction temperature is controlled at 40-50°C, and the reaction is kept for 1 hr. After cooling down to room temperature, extract with methyl tert-butyl ether, 100ml each time, extract three times in total, combine the obtained org...
Embodiment 3
[0034] Step 1: Add 300g of acetone to a 500mL flask, then add 89.2g (0.4mol) of permethrin methyl ester, cool down to -20°C, and start to feed ozone with a volume concentration of 8% at a flow rate of 0.5L / min for reaction ,, during the reaction process, the gas spectrum is used to analyze the raw materials in the central control, and after the conversion rate of methyl permethrin is greater than 99%, the ozone is stopped. After the reaction, add water, wash and separate the oil layer with water, and concentrate the oil layer to remove the solvent, then add 70g of 30% liquid caustic soda dropwise to the above reaction-finished material to carry out saponification reaction. At this time, the pH is greater than 12. Insulate and react at ℃ for 2 hours, then add 30% hydrochloric acid dropwise to the reaction solution to acidify the reaction until the pH value reaches 1-2, control the reaction temperature at 40-50 ℃, and insulate for 1 hr. After cooling down to room temperature, ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com