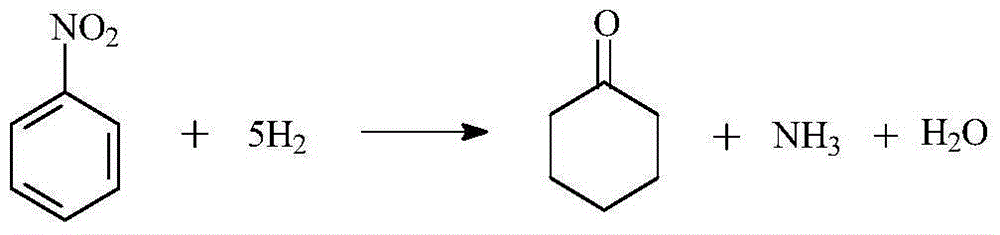
A kind of method directly synthesizing cyclohexanone by hydrogenation of nitrobenzene
A technology for nitrobenzene and cyclohexanone, applied in the field of chemical technology, can solve the problems of complex process route, low cyclohexene efficiency and high production cost, and achieve the effects of simple synthesis process, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In the autoclave, add nitrobenzene 0.49g successively, the Pd / MCM-41 (molecular sieve) catalyst 0.78g that loading capacity is mass percent 2%, AlCl 3 0.54g, cetyltrimethylammonium bromide 0.006g and water 10ml (the molar ratio is 1:0.037:1:0.004:139). Pass N 2 Make a replacement. Then the temperature was raised to 80°C, and then hydrogen gas was introduced to the reaction pressure of 1.3 MPa and maintained. After 1 hour of reaction, the hydrogen gas flow was stopped and the temperature was lowered to room temperature. The catalyst and the reaction solution were separated by filtration under reduced pressure, and the reaction solution was extracted and separated by toluene to obtain an organic phase, which was analyzed by gas chromatography. The yield of the product cyclohexanone was quantitatively calculated to be 56.7%.
Embodiment 2~5
[0035] The same operation steps and reaction conditions as in the process of synthesizing cyclohexanone in Example 1, the active metals of the catalyst were changed to Ru, Rh, Pt, Au respectively. The organic phase was analyzed by gas chromatography, and the yield of the product cyclohexanone was quantitatively calculated. The experimental results are shown in Table 1.
[0036] The impact of table 1 catalyst active metal on the synthetic cyclohexanone reaction
[0037] Example
Embodiment 6~10
[0039] The operating steps and reaction conditions of the synthetic cyclohexanone process in Example 1 are the same, and the carrier of the catalyst is changed to AC, γ-Al 2 o 3 , TS-1 (molecular sieve), HZSM-5 (molecular sieve), SBA-15 (molecular sieve), etc. The organic phase was analyzed by gas chromatography, and the yield of the product cyclohexanone was quantitatively calculated. The experimental results are shown in Table 2.
[0040] The impact of table 2 catalyst carrier on the synthetic cyclohexanone reaction
[0041] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com