Sealing Device And Delivery System
一种递送、医疗装置的技术,应用在密封装置领域,能够解决难以旋转等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
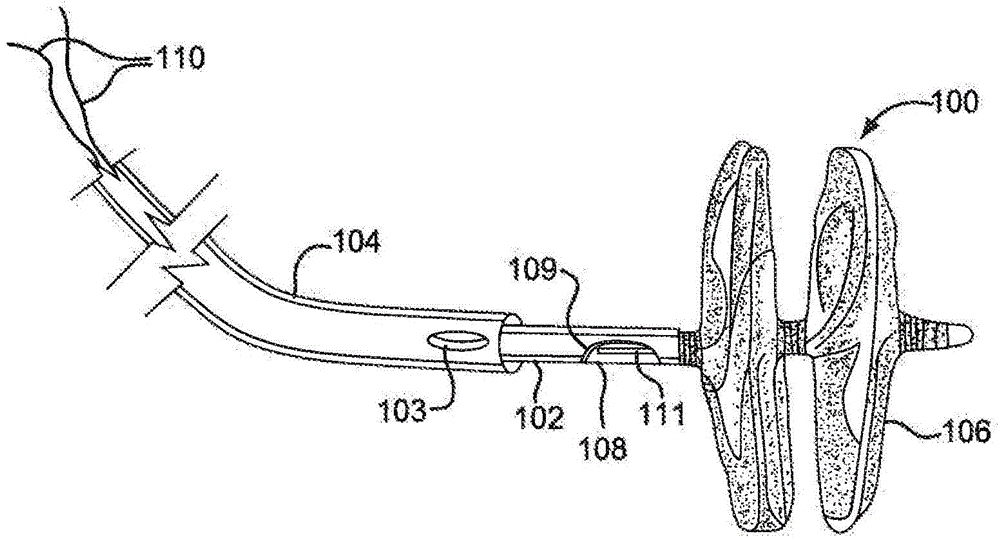
[0121] Use the following components and assembly process to fabricate a figure 1 sealing device.
[0122] An expanded polytetrafluoroethylene material with the following properties is obtained:
[0123] The bubble point of methanol is 1psi (pounds per square inch)
[0124] Mass / area of 2.2 g / m²
[0125] Maximum longitudinal load is 1.6 kg / in
[0126] 0.0003 inches thick
[0127] Longitudinal matrix tensile strength of 92,000 psi (pounds per square inch)
[0128] The following test method and setup were used to determine the above properties: The methanol bubble point was measured using a custom machine with a 1 inch diameter foot, a ramp rate of 0.2 psi / sec, and a liquid medium of methanol. Use a metal ruler to measure the length and width of the material. Mass / area was measured on 36 x 5 inch samples using a balance (ANG, Model GF-400 Top Loading Balance, San Jose, CA). The longitudinal maximum load was measured using a materials testing machine (Instron Model 5564, ...
example 2
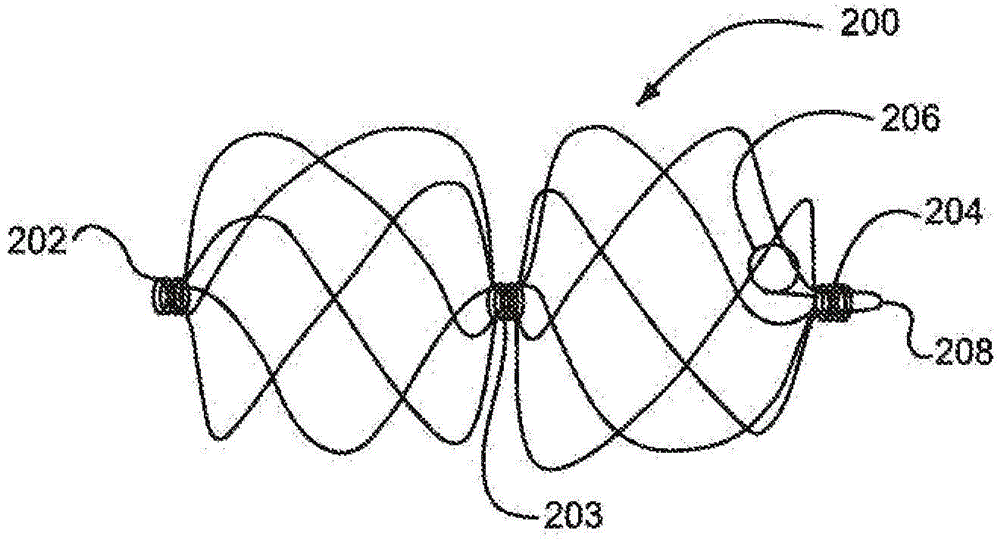
[0148] Use the following components and assembly process to fabricate a Figure 6 sealing device.
[0149] Expanded polytetrafluoroethylene and expanded polytetrafluoroethylene with a thin layer of FEP (fluorinated ethylene propylene) material similar to that described in Example 1 were obtained.
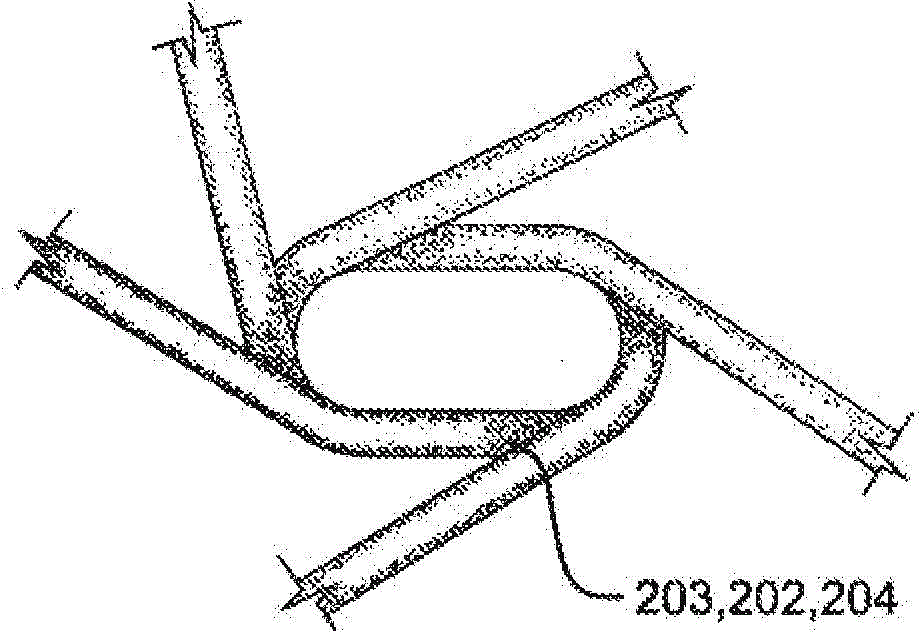
[0150] The distal eyelet was formed by first obtaining a length of 10% platinum pull-filled Nitinol wire (Fort Wayne Metals, Fort Wayne, IN) approximately 0.23 mm in diameter. This line is labeled "First Line". The free end of the first thread is folded over itself to form an open-ended loop, and the open-ended loop is inserted into the button. The button is then inserted onto the keyed center pin. The button is shaped with an opening through the center to accommodate the keyed center pin and has features that allow it to rest securely within the winding jig. A keyed center pin (major axis about 5.79 mm and minor axis about 0.25 mm and length about 10.16 mm) was inserted into th...
example 3
[0160] Use the following components and assembly process to fabricate a Figure 8 handle components.
[0161] An injection molding process is used to make the parts of the handle assembly. Parts used by ContourPlastic (Baldwin, WI) 348 to make. This material is suitable for use in medical devices and exhibits an impressive tensile strength of 48.2 MPa and tensile modulus of 2.62 GPa. using injection molding process and 348 to make nine parts. These parts include the Second Linear Actuator, Flush Pad Holder, First Linear Actuator, Retraction Cord Lock, Spindle Control Lever, Left Body Housing, Mounting, Right Body Housing, and Lock Release Actuator device.
[0162] Other materials needed to assemble the handle are commercially available items. Catheters formed with a hand lay-up process generally known in the art (Teleflex Medical, Jeffrey, NH) had an outer diameter of 0.33 mm and an inner diameter of 0.048 mm, with a platinum-iridium marker band placed on the distal t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com