Indanone compound dual-targeting to ovarian cancer cell tubulin and peripheral blood vessels thereof, and synthetic method and application thereof
A technology of tubulin and compounds, applied in the field of biomedicine, can solve problems such as poor water solubility, high incidence of drug resistance, and narrow anti-tumor spectrum, and achieve the effects of small molecular weight, inhibition of proliferation, and high anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
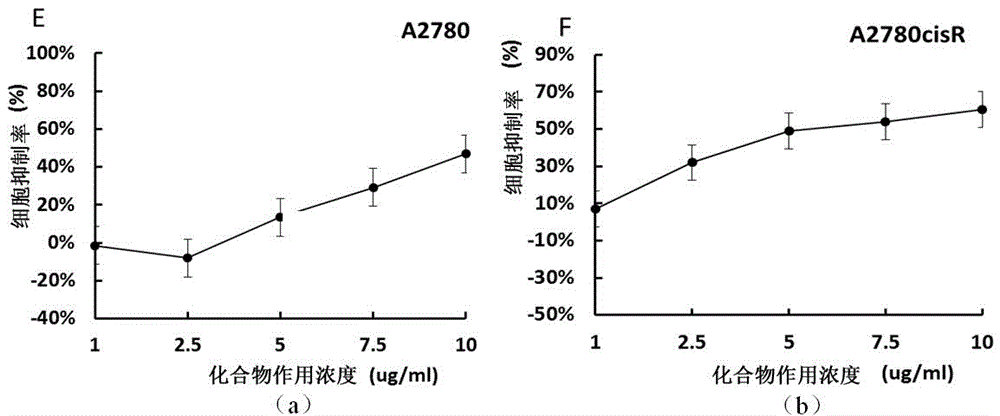
[0039] Example 1 CCK-8 method detects cell viability and confirms the compound 1 For ovarian cancer A2780, A2780cisR has an anti-tumor effect, see figure 1 shown. By CCK-8 method, we tested the cell viability of A2780 and A2780cisR cells treated with compound 1. It was found that compound 1 can inhibit the proliferation of ovarian cancer cells, and this inhibitory effect is concentration-dependent. Among them, at the concentration of 10 μg / mL, the tumor inhibition rate of the cells was 49.3%. At the same time, according to the tumor cell growth inhibition curve, the IC50 of compound 1 against A2780 and A2780cisR were 5.48 and 5.98 μg / mL, respectively.
Embodiment 2
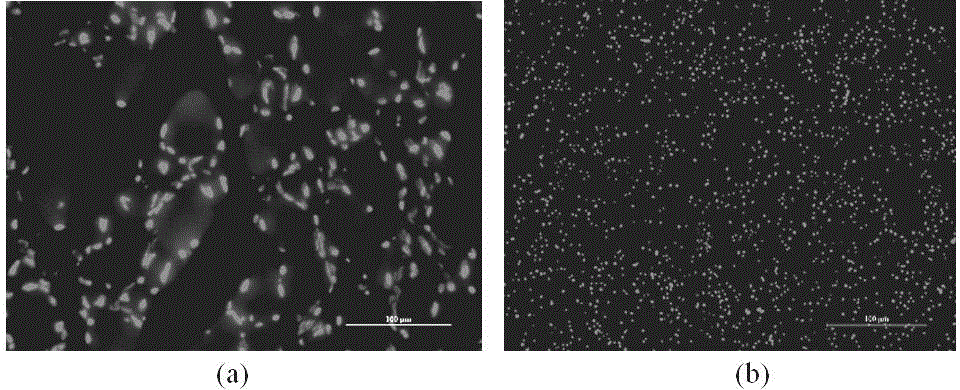
[0040] Example 2 Hochest staining method to detect the apoptosis rate confirms the compound 1 It can promote the apoptosis of ovarian cancer SKOV3. See figure 2 shown. The apoptotic ability of ovarian cancer SKOV3 treated with Compound 1 was analyzed by Hochest staining. Among them, compared with the blank control group, the number of apoptotic cells in the ovarian cancer A2780 and A2780cisR cells treated with compound 1 increased, and the numbers were (76.3±64.2) and (26.1±14.2) in turn. There is a statistical difference (P figure 2 It can be seen that a lot of nuclear fragmentation and nuclear condensation can be seen in the ovarian cancer cells treated with compound 1, and some nuclear accumulation can also be seen in it.
Embodiment 3
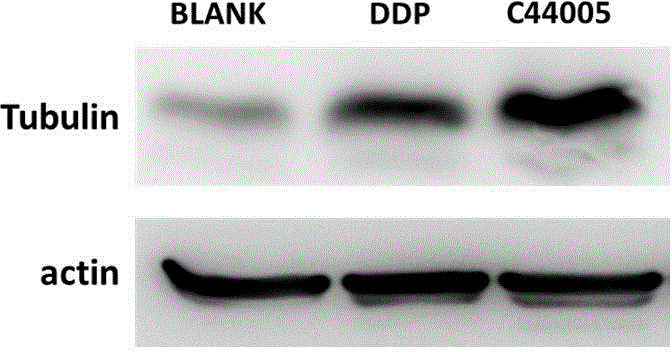
[0041] Example 3 The Western Blot method was used to detect the expression of tubulin α in the tumor tissues of each group, and it was confirmed that compound 1 has the effect of promoting tubulin aggregation and inhibiting its depolymerization on ovarian cancer SKOV3 cells in vivo, see image 3 shown. Tublinα is the main component of microtubules, so we detected the expression of Tublinα in each group of tumor tissues by Western Blot method. The results showed that there were differences in the expression of Tublinα in each group, and the expression of Tublinα in the tumor tissue of the nude mice in the compound 1 group was the highest.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com