Method, kit, stem cells and application for preparing autologous hematopoietic stem cells
A technology of hematopoietic stem cells and somatic cells, applied in the field of biomedicine, can solve the problems of low safety, less available quantity, insufficient sources of hematopoietic stem cells, etc., and achieve the effect of production speed and safety advantages.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of Example 1 Kit
[0060] Operate in a safe operation table with a cleanliness of 10-100, and prepare at a low temperature of 4-10 degrees. In 500ml of RPMI640 medium, add: 10μM Y-27632 (C14H21N3O 2HCl); 10ng / mL stem cell factor; 10ng / mL interleukin 3; 10ng / mL interleukin 6; 10ng / mL interleukin 11; 10ng / mL macrophage colony stimulating factor (M-CSF); 10ng / mL granulocyte colony stimulating factor (G-CSF); 10 μg / mL fucoidan; 10 μg / mL sulfuric acid Dextran; 10 nM AMD3100 (1,1′-[1,4-phenylenebis(methylene)]-di-1,4,8,11-tetraazacyclotetradecane); 10 μM M-alendronate sodium trihydrate; 10 μM disodium pamidronate.
[0061] Mix well, dissolve, and then filter and sterilize through a 0.22-micron pore size filter to prepare a cell culture solution.
[0062] The cell culture fluid is placed in the kit to prepare a kit for producing protein-induced autologous hematopoietic stem cells.
Embodiment 2
[0063] Example 2 Preparation of protein-induced autologous hematopoietic stem cells (M-PiHPS) in mice
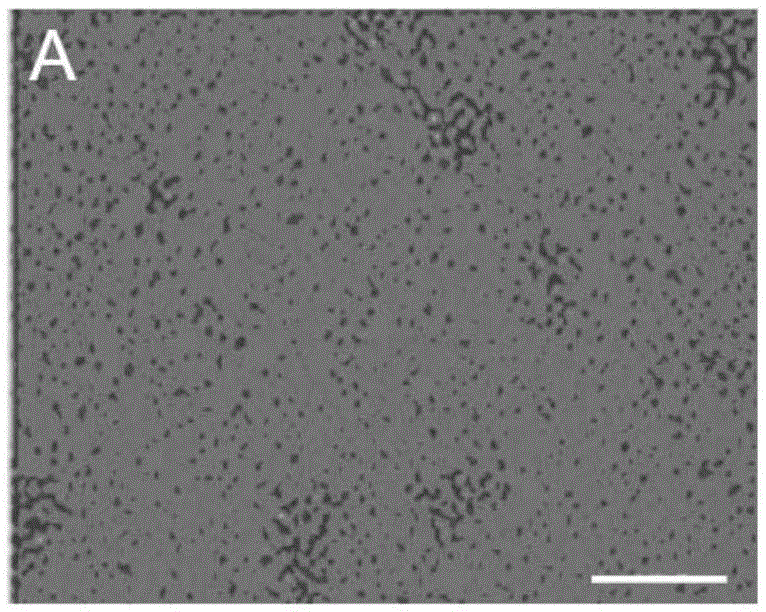
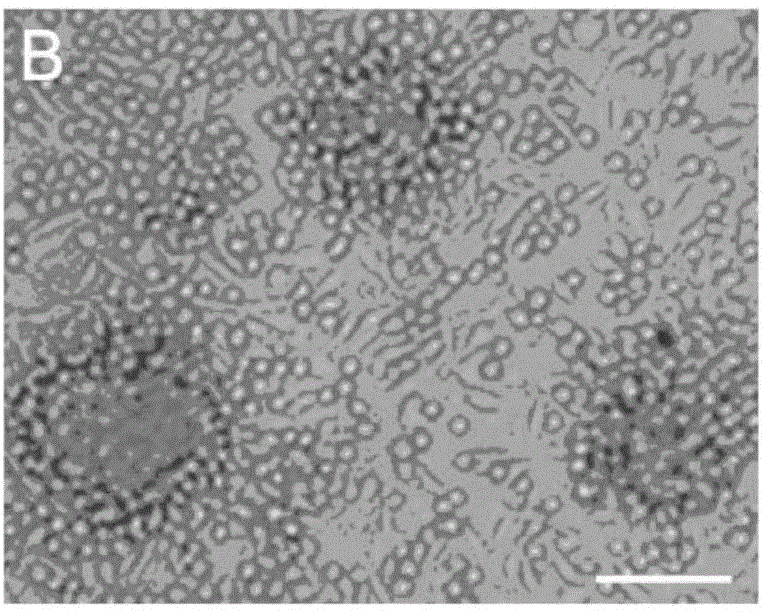
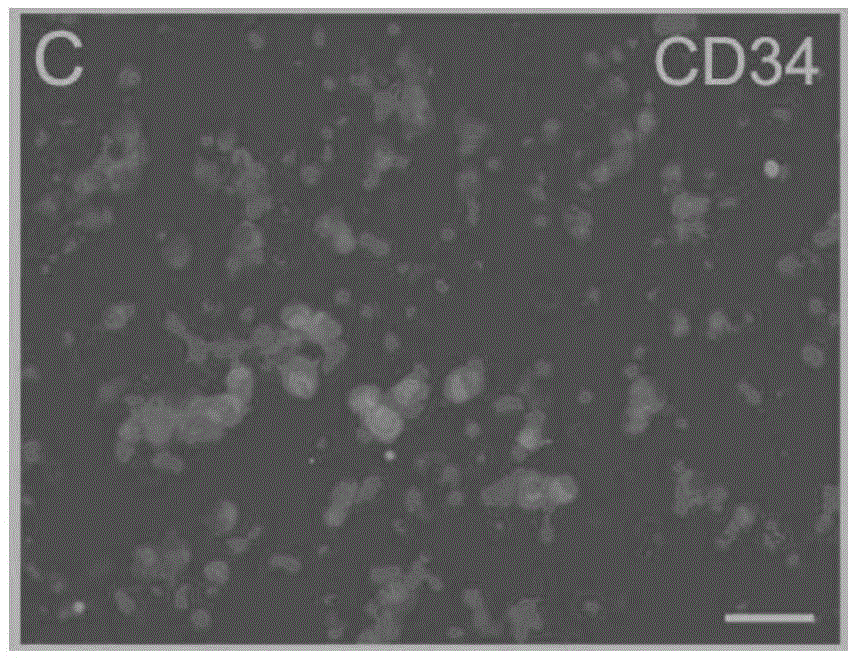
[0064] 3-5 month old Balb / C male mice (purchased from School of Radiology, Chinese Academy of Medical Sciences (Tianjin), animal certificate number: SCXK (Tianjin) 2010-0002) were selected, and all their blood was collected and centrifuged using conventional Ficoll The technology centrifuges and separates blood to obtain blood mononuclear cells. The obtained mononuclear cells were divided into 5 × 10 6 The density of cells / ml was inoculated in a cell culture dish, and the cell culture solution prepared in Example 1 was added to it, at 37°C and 5% CO. 2 Incubator for 5 days to obtain protein-induced autologous hematopoietic stem cells. Gently pipetting the cultured cells to completely suspend the cells, collect the cells in a centrifuge tube, and perform the centrifugation-physiological saline suspension cell washing operation. Sexual autologous hematopoietic stem cells. ...
Embodiment 3
[0065] Example 3 Preparation of human protein-induced autologous hematopoietic stem cells (M-PiHPS)
[0066] 50ml of human peripheral blood was collected, and the blood was centrifuged and separated using conventional Ficoll centrifugation technology to obtain blood mononuclear cells. The obtained mononuclear cells were divided into 5 × 10 6 The density of cells / ml was inoculated in a cell culture dish, and the cell culture solution prepared in Example 1 was added to it, at 37°C and 5% CO. 2 Incubator for 5 days to obtain protein-induced autologous hematopoietic stem cells. Gently pipetting the cultured cells to completely suspend the cells, collect the cells in a centrifuge tube, and perform the centrifugation-physiological saline suspension cell washing operation. Sexual autologous hematopoietic stem cells. The obtained protein-induced autologous hematopoietic stem cells were 1 × 10 5 pcs / ml to 1×10 6 The cell density of cells / ml was mixed with an equal volume of a mixt...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap