A new 19-demethylbufolide compound and its application in the preparation of antitumor drug preparations
A technology for demethylation toad and anti-tumor drugs, applied in the field of natural medicine and chemical medicine, can solve the problems of complex chemical composition of toad skin, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
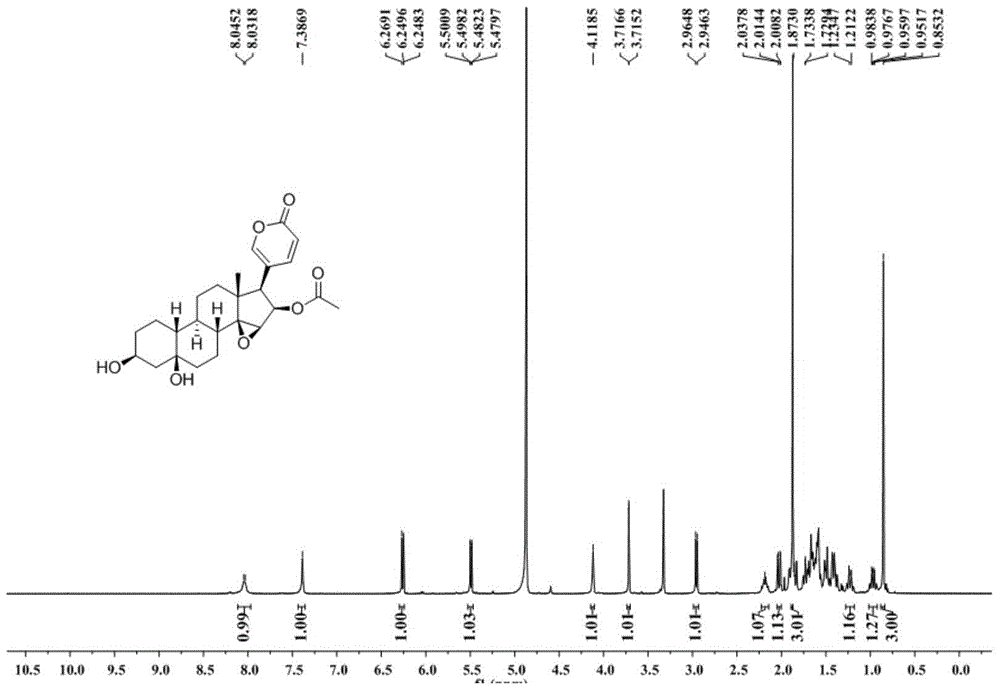
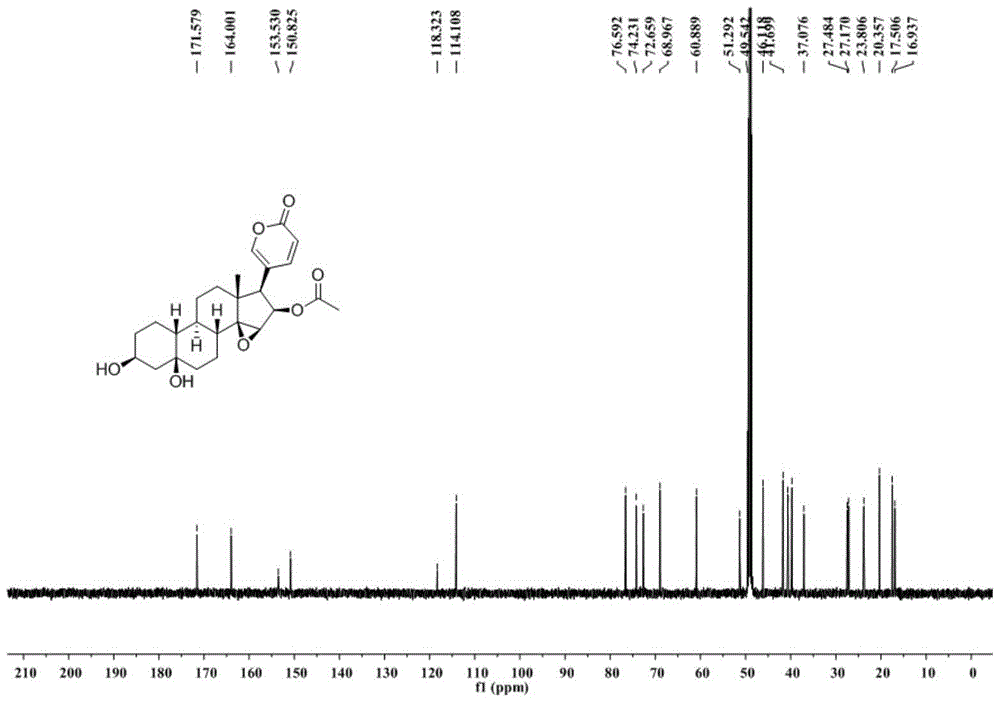
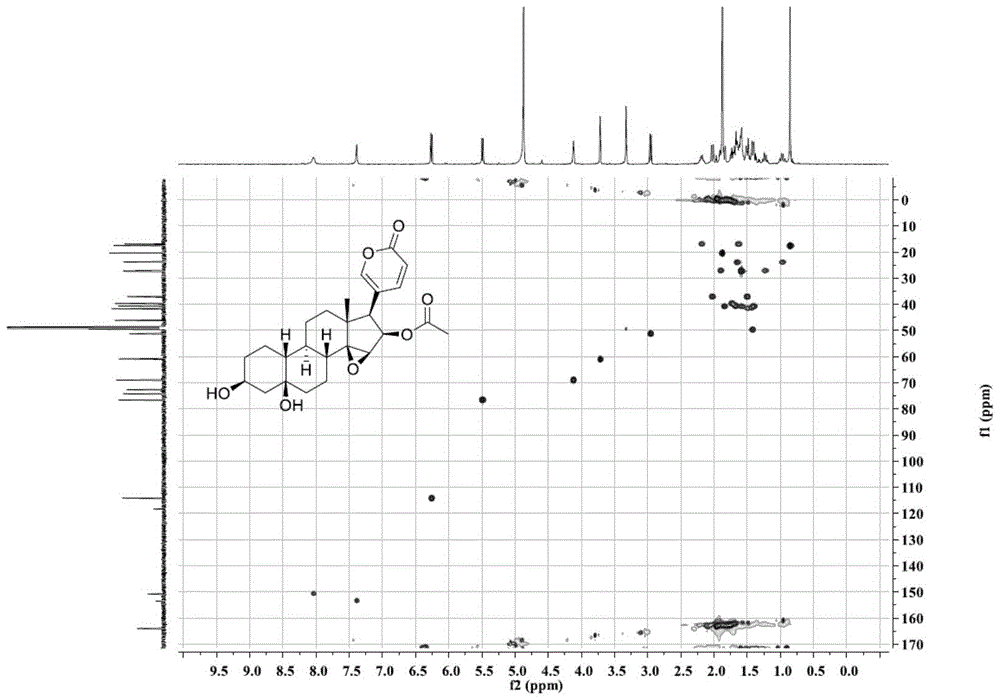
[0024] Extraction, separation and structural identification of the compound of Example 1
[0025] (1) Take 11 kg of dried toad skin, grind it into 10-50 mesh toad skin coarse powder with a pulverizer, heat and reflux extraction with 3 times the mass of distilled water at 100°C for 4 times, 1 hour each time; combine the extracts and decompress Concentrate to a density of 1.15, add ethanol and mix evenly to make the alcohol content 80% (v / v), let it stand for 24 hours, absorb the supernatant, concentrate under reduced pressure to obtain the water extract; then extract the toad skin powder after water extraction The residue was heated and refluxed with 3 times the mass of 95% (v / v) ethanol at 80°C to extract 4 times, 1 hour each time; the extracts were combined and concentrated under reduced pressure to obtain the ethanol extract; the total water extract and the total ethanol extract The mixtures were combined, suspended with 6L of water, extracted with 6L of chloroform, and repe...
Embodiment 2
[0036] Example 2 Inhibitory Effect of Bufogargarin A on Tumor Cell Proliferation
[0037] Cells in logarithmic growth phase (human liver cancer cell HepG2, human non-small cell lung cancer cell A549, human cervical cancer cell Hela, human colon cancer cell Lovo, human breast cancer cell MCF-7, human esophageal cancer cell Eca-109) (All cell lines were purchased from American Type Culture Collection, ATCC) Digested with trypsin, centrifuged, resuspended, and counted cells at 1×10 6 / mL inoculated in a 96-well culture plate, 100 μL per well, after the cells adhered to the wall, dilute the drug to be tested into a certain concentration gradient, set 4 parallel replicate wells for each concentration, and set a blank control group and a positive control group at the same time (Doxorubicin), placed in a constant temperature incubator (37 ° C, 5% CO 2Cultured in medium for 72h. Aspirate off the medium, add 30 μL of MTT solution (5 mg / mL) to each well, continue to incubate for 4 h, ...
Embodiment 3
[0041] Example 3 Anti-tumor effect of Bufogargarin A in vivo
[0042] Test method: Kunming mice weighing 18-22 g (purchased from Guangdong Provincial Animal Center) were randomly divided into groups of 10 mice. Tumor cells (S180 ascites tumor cells) were inoculated in the armpits of their right forelimbs with 2×10 7 After 24 hours, intraperitoneal injection was administered once a day, and the animals were sacrificed after 10 days of continuous administration, and the tumors were taken and weighed.
[0043] The tumor inhibition rate was calculated according to the following formula:
[0044] Tumor inhibition rate (%)=[1-(average tumor weight of administration group) / average tumor weight of control group]×100%
[0045] Statistical analysis was processed by t test, and P<0.05 was considered to have significant difference. The results are shown in Table 3.
[0046] Table 3. The inhibitory effect of Bufogargarin A on the growth of mouse S180 solid tumor (n=10)
[0047]
[...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap