Application of a α-l-rhamnosidase in the preparation of 5-fluoro-2'-deoxyuridine derivatives
A technology of deoxyuridine and deoxyuridine mice, which is applied in the field of glycoengineering, can solve the problems of low selectivity, toxic and side effects, limited, etc., and achieves the effects of increasing stability and targeting, and having broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of 5-fluoro-2'-deoxyuridine rhamnoside, the steps are as follows:
[0048] 1. Preparation of α-L-rhamnosidase
[0049] Artificially synthesize the α-L-rhamnosidase sequence with GenBank accession number JN704640 (encoded protein GenBank accession number is AFA41506.1, nucleotide sequence such as SEQ ID NO.2), connect it to the pPIC9K plasmid, and transform Pichiapastoris GS115. The α-L-rhamnosidase was prepared according to the Pichia pastoris expression operation manual of Invitrogen, and the protein content of the enzyme was determined by the Coomassie brilliant blue method. After detection, the amino acid sequence was shown in SEQ ID NO.1.
[0050] 2. Synthesis of 5-fluoro-2'-deoxyuridine rhamnoside catalyzed by α-L-rhamnosidase
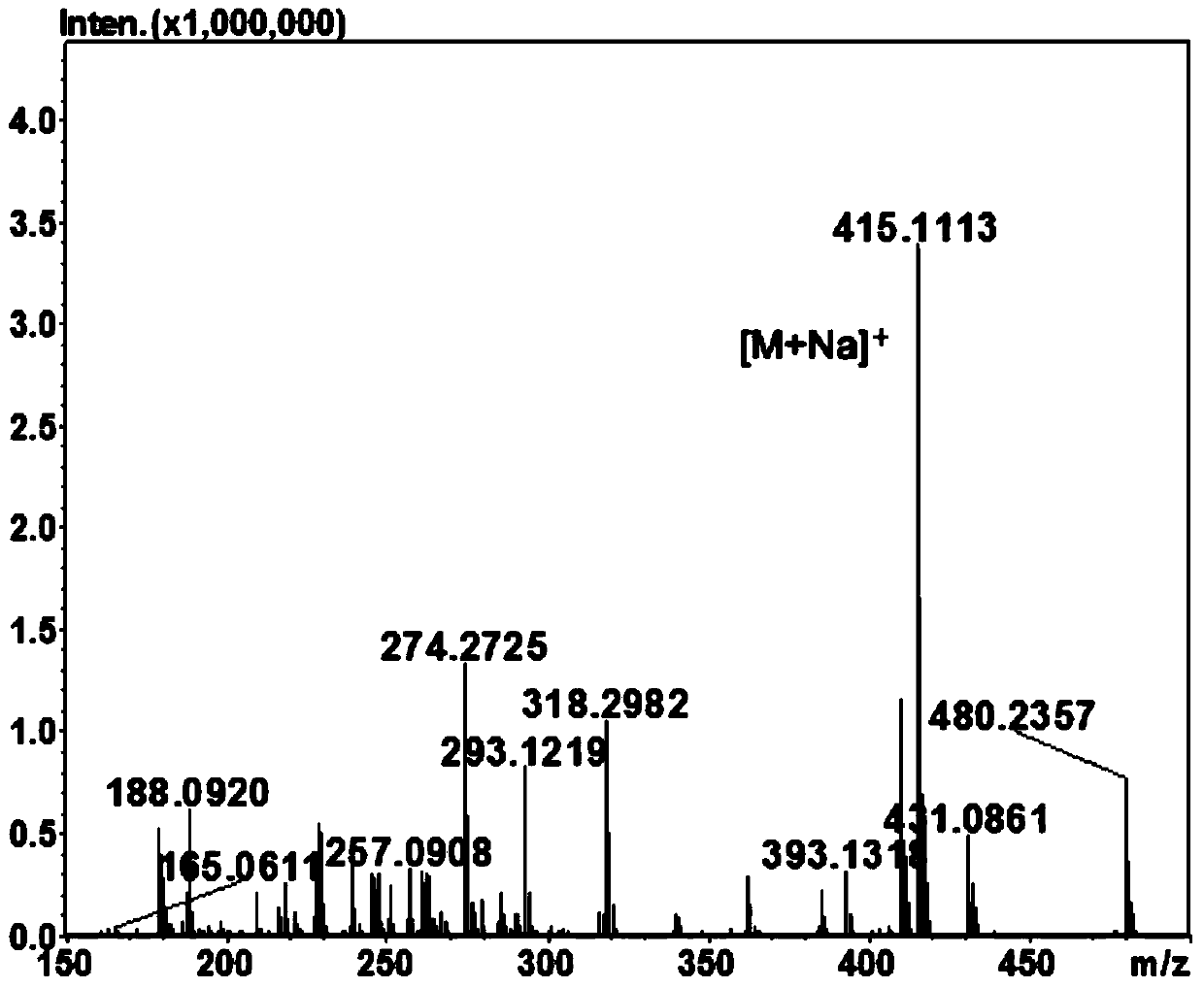
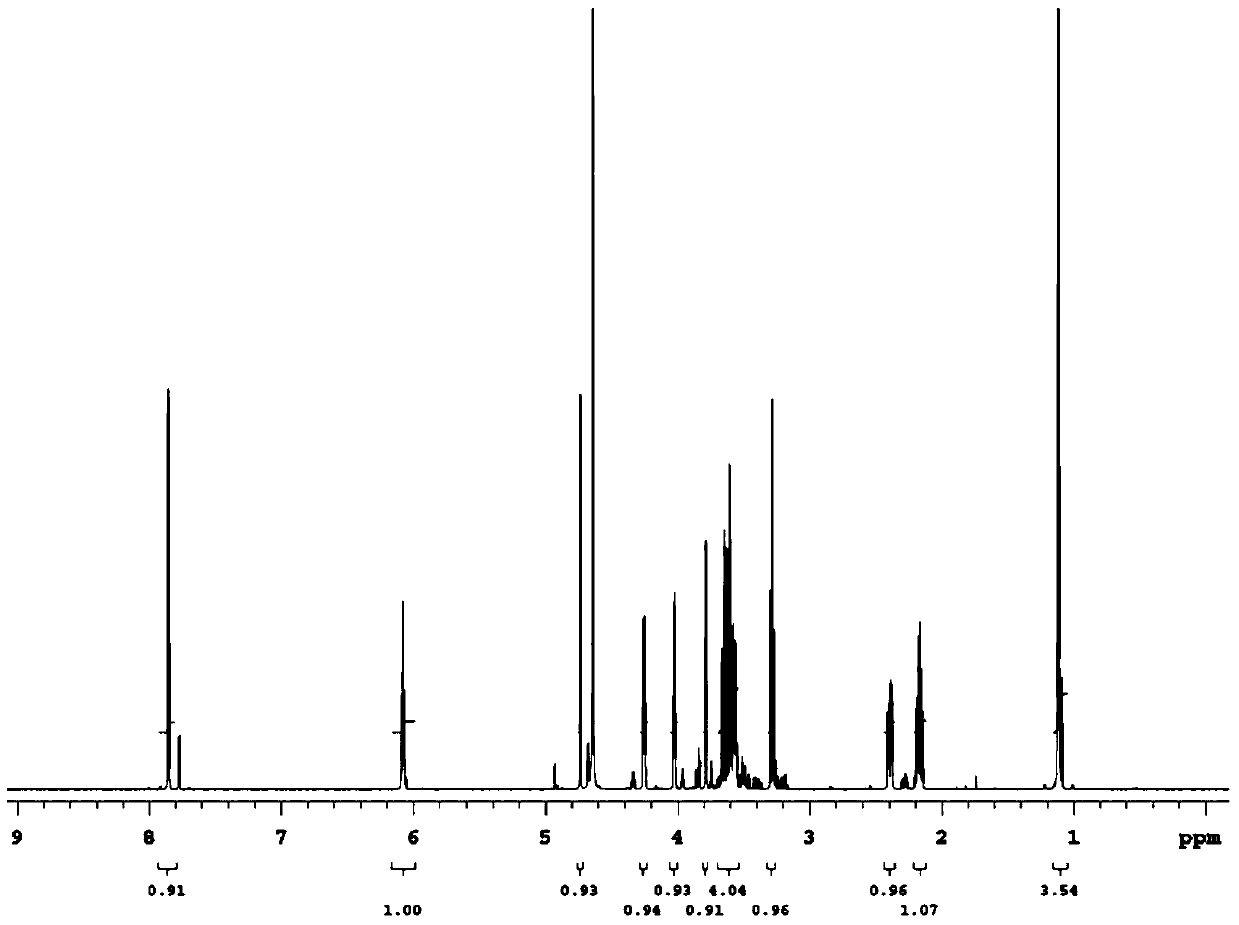
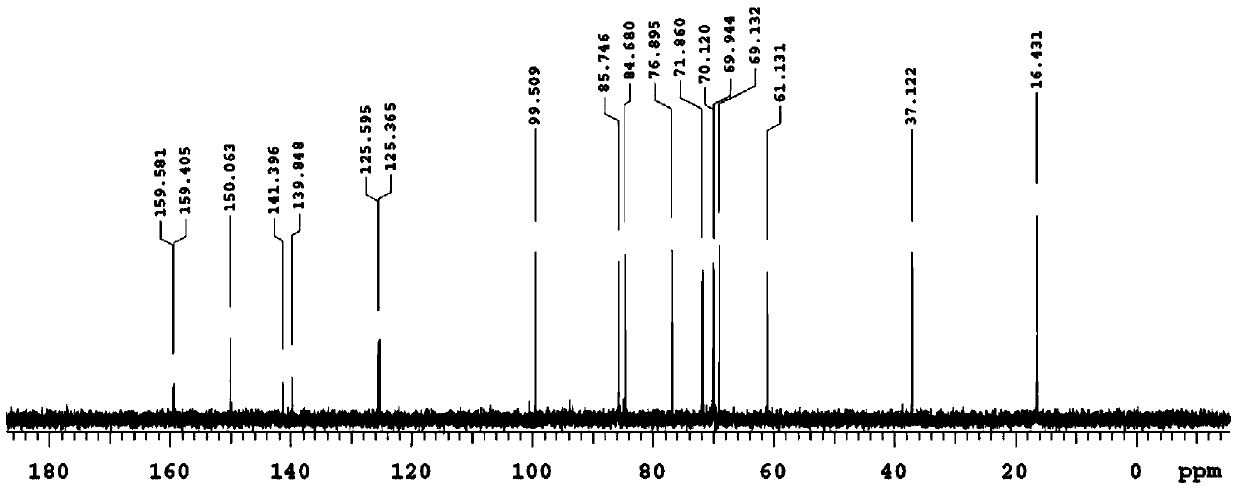
[0051] 10 mL of the reaction system was prepared with pH 6.5, 50 mM sodium phosphate buffer solution, the final concentration of rhamnose was 0.4 M, and the amount of enzyme added was 8 μg / mL. After reacting at 55°...
Embodiment 2
[0063] A method for enzymatically preparing 5-fluoro-2'-deoxyuridine rhamnoside, comprising the steps of:
[0064] (1) Use phosphate buffer to prepare α-L-rhamnosidase with a rhamnose concentration of 0.3M, a 5-fluoro-2'-deoxyuridine concentration of 0.4M, and an amino acid sequence as shown in SEQ ID NO.1 The reaction system with an addition amount of 5 μg / mL;
[0065] The phosphate buffer in the step (1) is a sodium phosphate buffer with a concentration of 10mM and pH6;
[0066] (2) React the reaction system prepared in step (1) in a water bath at 45°C for 40 hours, boil at 100°C for 5 minutes to terminate the reaction, centrifuge at 10,000 rpm for 30 minutes, and take the supernatant;
[0067] (3) The supernatant obtained in step (2) is separated and detected by thin-layer chromatography, and the product with the same migration distance and color development under ultraviolet light is freeze-dried to obtain 5-fluoro-2' - deoxyuridine rhamnoside;
[0068] The separation i...
Embodiment 3
[0074] A method for enzymatically preparing 5-fluoro-2'-deoxyuridine rhamnoside, comprising the steps of:
[0075] (1) Use phosphate buffer to prepare α-L-rhamnosidase with a rhamnose concentration of 0.5M, a 5-fluoro-2'-deoxyuridine concentration of 0.6M, and an amino acid sequence as shown in SEQ ID NO.1 The reaction system with an addition amount of 10 μg / mL;
[0076] The phosphate buffer in the step (1) is a sodium phosphate buffer with a concentration of 100mM and a pH of 8;
[0077] (2) React the reaction system prepared in step (1) in a water bath at 60°C for 60 hours, boil at 100°C for 5 minutes to terminate the reaction, centrifuge at 12,000 rpm for 30 minutes, and take the supernatant;
[0078] (3) The supernatant obtained in step (2) is separated and detected by thin-layer chromatography, and the product with the same migration distance and color development under ultraviolet light is freeze-dried to obtain 5-fluoro-2' - deoxyuridine rhamnoside;
[0079] The sepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com