Application of alpha-L-rhamnosidase to preparing hydroxycarbamide and glycoside derivatives
A rhamnosidase amino acid and hydroxyurea glycoside technology, which is applied in the field of glycoengineering, can solve the problems of no rhamnosylation modification of hydroxyurea, and achieve the goal of improving anti-tumor activity, increasing targeting, and reducing toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
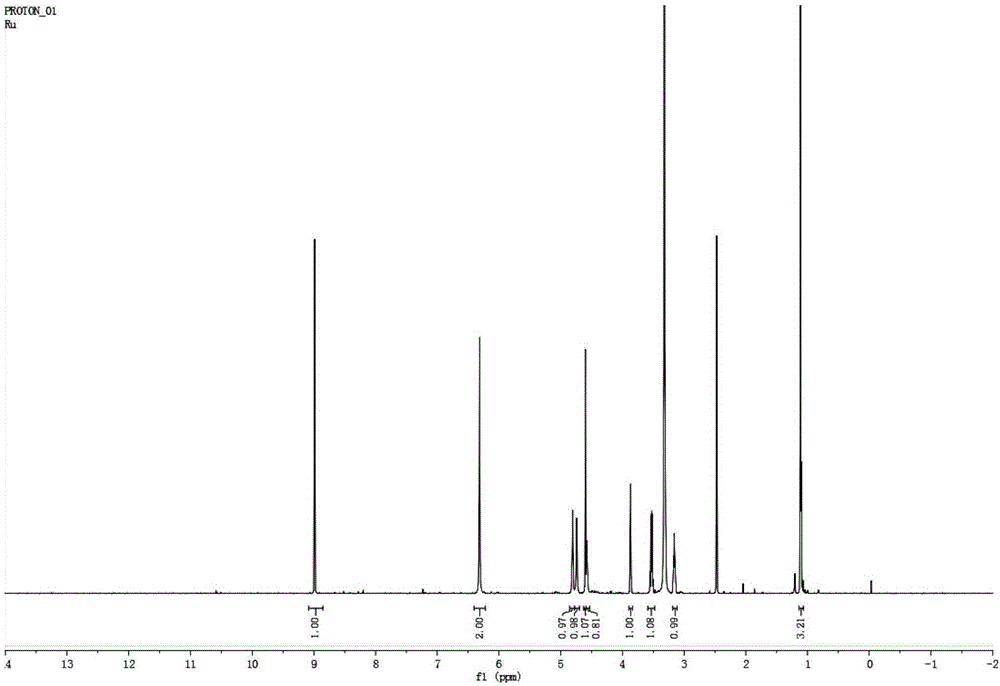
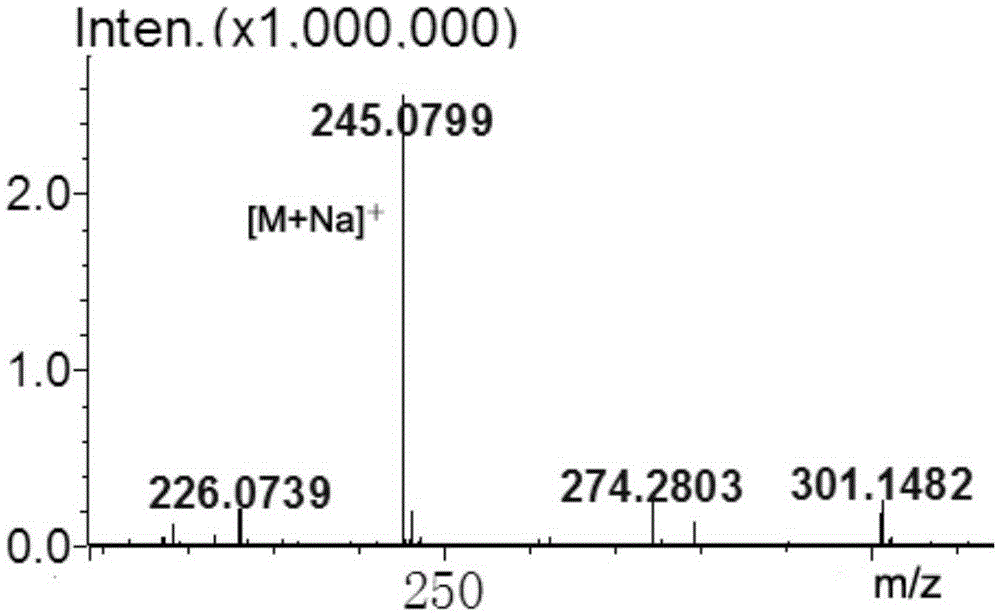
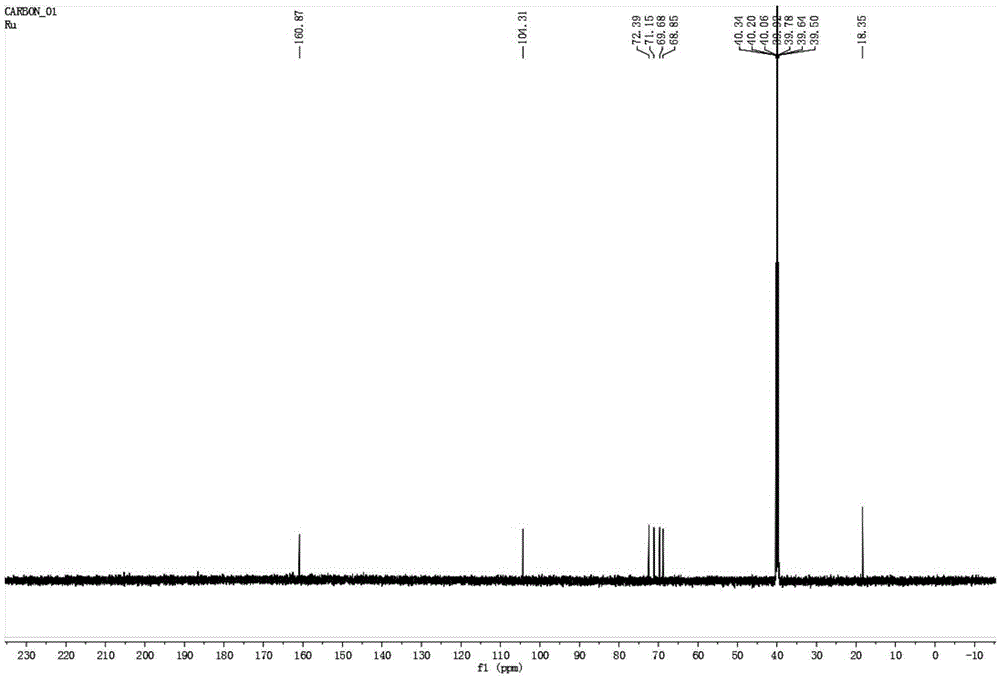
[0039] Novel hydroxyurea rhamnoside derivatives, the preparation steps are as follows:
[0040] 1. Preparation of α-L-rhamnosidase
[0041] The α-L-rhamnosidase sequence with GenBank accession number JN704640 (encoded protein GenBank accession number is AFA41506.1, nucleotide sequence as SEQ ID NO.2) was artificially synthesized, ligated to pPIC9K plasmid, and transformed into PichiapastorisGS115. The α-L-rhamnosidase was prepared according to the instructions of the Pichia pastoris expression operation manual provided by Invitrogen, and the content of the enzyme protein was determined by the Coomassie brilliant blue method. After detection, the amino acid sequence was shown in SEQ ID NO.1.
[0042] 2. α-L-rhamnosidase catalyzes the synthesis of hydroxyurea rhamnoside
[0043] 1 mL of the reaction system was prepared with pH 6.5, 50 mM potassium sodium phosphate buffer solution, the final concentration of rhamnose was 0.4 M, the final concentration of hydroxyurea was 0.4 M, a...
Embodiment 2
[0052] A method for enzymatically preparing hydroxyurea rhamnoside, comprising the steps of:
[0053] (1) Use sodium phosphate buffer to prepare a reaction system in which the concentration of rhamnose is 0.3M, the concentration of hydroxyurea is 0.3M, and the amount of α-L-rhamnosidase shown in SEQ ID NO.1 is 5 μg / mL. ;
[0054] The buffer in the step (1) is a sodium phosphate buffer with a concentration of 10mM and pH6;
[0055] (2) React the reaction system prepared in step (1) in a water bath at 45°C for 24 hours, boil at 100°C for 5 minutes to terminate the reaction, centrifuge at 10,000 rpm for 30 minutes, and take the supernatant;
[0056] (3) Separate the supernatant obtained in step (2) with a Bio-gelP2 chromatographic column with a specification of 15mm×100cm, use water as the mobile phase, and a flow rate of 0.3mL / min, collect the eluted samples, and detect them by thin-layer chromatography , combine products with the same migration distance, freeze-dry and make pow...
Embodiment 3
[0061] A method for enzymatically preparing hydroxyurea rhamnoside, comprising the steps of:
[0062] (1) Use sodium phosphate buffer to prepare a reaction system in which the concentration of rhamnose is 0.5M, the concentration of hydroxyurea is 0.5M, and the amount of α-L-rhamnosidase shown in SEQ ID NO.1 is 10 μg / mL. ;
[0063] The buffer in the step (1) is a sodium phosphate buffer with a concentration of 100mM and a pH of 8;
[0064] (2) React the reaction system prepared in step (1) in a 60°C water bath for 48 hours, boil at 100°C for 5 minutes to terminate the reaction, centrifuge at 12000 rpm for 30 minutes, and take the supernatant;
[0065] (3) Separate the supernatant obtained in step (2) with a Bio-gelP2 chromatographic column with a specification of 15mm×100cm, use water as the mobile phase, and a flow rate of 0.3mL / min, collect the eluted samples, and detect them by thin-layer chromatography , combine products with the same migration distance, freeze-dry and ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com