Alkaline low-calcium amino acid (15) peritoneal dialyzate medicament composition
A peritoneal dialysate, composition technology, applied in the direction of drug combination, alkali/alkaline earth metal chloride active ingredients, blood diseases, etc., can solve the problem of drug effect, stability, irritating adverse reactions can not have both, increase the generation of insoluble particles , increase the risk of patients, etc., to reduce metabolic burden, avoid adverse reactions, and correct amino acid spectrum disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0040] Each liter of peritoneal dialysis fluid consists of the following components
[0041]
[0042] Preparation:
[0043] Concentrated solution preparation: Take 20% of the prescription amount of water for injection, add other ingredients of the prescription amount and stir until completely dissolved, then add medicinal charcoal 0.1-1%, stir evenly, leave it for 10-20 minutes, and filter through a 0.22μm terminal Steam sterilize at 115°C for 10 minutes to obtain a spare concentrate.
[0044] 2. Preparation of lye: Prepare a tris solution with a content of 0.1mol / L, then add 0.1-1% medicinal charcoal and stir evenly, leave it for 10-20 minutes, filter through a 0.22μm terminal, and steam at 115°C Sterilize for 10 minutes to obtain lye for later use.
[0045] 3. Mix the concentrated solution and lye at a constant speed, adjust the solution to 1000ml through lye and water for injection, and the pH value is the value in the above table, and then filter through a 0.22μm term...
Embodiment 4-6
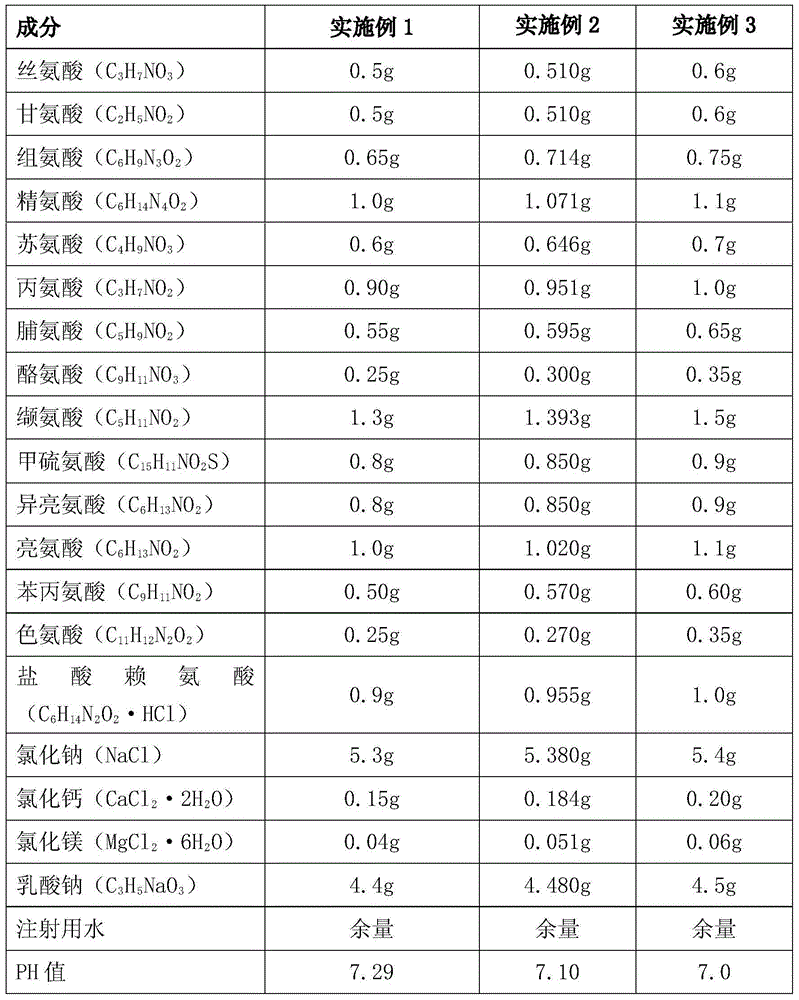
[0052] Each liter of peritoneal dialysis fluid consists of the following components
[0053] Element Example 4 Example 5 Example 6 Serine (C 3 h 7 NO 3 ) 0.5g 0.510g 0.6g Glycine (C 2 h 5 NO 2 ) 0.5g 0.510g 0.6g Histidine (C 6 h 9 N 3 o 2 ) 0.65g 0.714g 0.75g Arginine (C 6 h 14 N 4 o 2 ) 1.0g 1.071g 1.1g Threonine (C 4 h 9 NO 3 ) 0.6g 0.646g 0.7g Alanine (C 3 h 7 NO 2 ) 0.90g 0.951g 1.0g Proline (C 5 h 9 NO 2 ) 0.55g 0.595g 0.65g Tyrosine (C 9 h 11 NO 3 ) 0.25g 0.300g 0.35g Valine (C 5 h 11 NO 2 ) 1.3g 1.393g 1.5g Methionine (C 15 h 11 NO 2 S) 0.8g 0.850g 0.9g Isoleucine (C 6 h 13 NO 2 ) 0.8g 0.850g 0.9g Leucine (C 6 h 13 NO 2 ) 1.0g 1.020g 1.1g Phenylalanine (C 9 h 11 NO 2 ) 0.50g 0.570g 0.60g Tryptophan (C 11 h 12 N 2 o 2 ) 0.25g 0.270g 0.35g Lysine hydrochloride 0.9g 0.9...
Embodiment 1-3
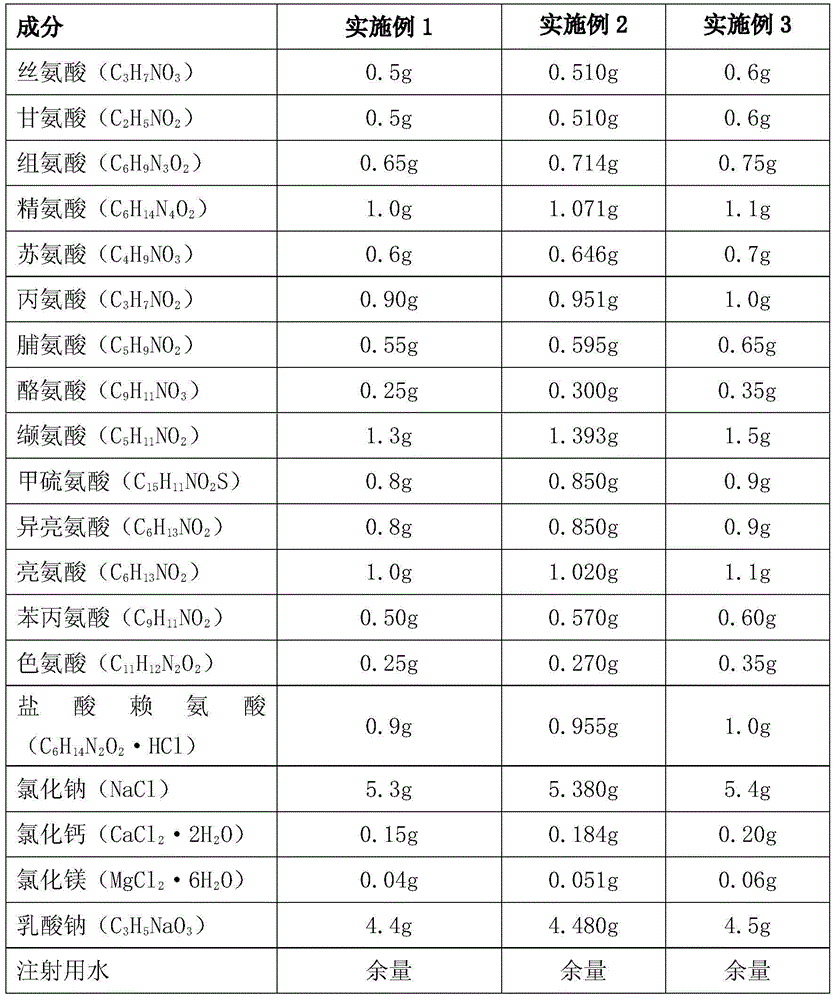
[0059] Each liter of peritoneal dialysis fluid consists of the following components
[0060]
[0061]
[0062] Preparation:
[0063] 1. Add 80% of the prescription amount of fresh water for injection (70-80°C) into the liquid mixing tank, feed the ingredients according to the order of the prescription under nitrogen flow, and stir to dissolve.
[0064] 2. When the temperature of the medicinal solution drops to room temperature, adjust it to 6≤PH<7 with hydrochloric acid, and replenish water to the full amount.
[0065] 3. Add activated carbon 0.1% (w / v), stir for about 30 minutes, and decarbonize.
[0066] 4. Filter with a φ0.45μm + φ0.22μm double-layer microporous membrane until the liquid medicine is clear, fill it with nitrogen, and stopper.
[0067] 5. Sterilize at 118°C for 15 minutes.
[0068] 6. Full inspection and packaging.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap