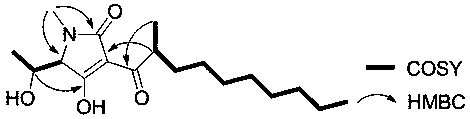
Penem enol e1 derived from Trichoderma aurantii and its application in the preparation of anti-oral epidermal cancer drugs
A technology of Trichoderma citrinum and penemol, which is applied in the field of medicinal chemistry, can solve problems such as drugs that have not yet been seen, and achieve the effect of significant anti-oral epidermal cancer activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
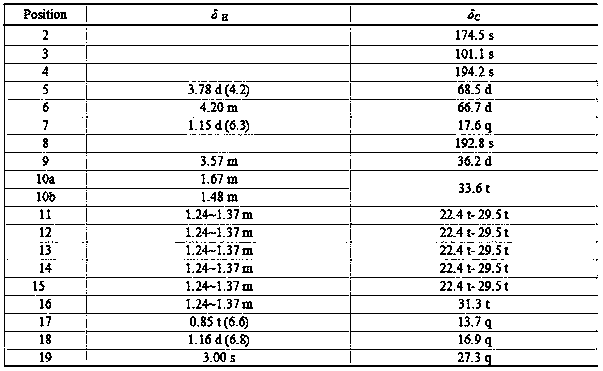
[0015] Fermentative production and separation and purification of the compound of embodiment 1
[0016] 1 Fermentation production
[0017] Fermentation culture of production bacteria: according to the conventional method of cultivating microorganisms, take Trichoderma auranthoderma ( Trichoderma citrinoviride. ) IBPT-4 (preserved in China Center for Type Culture Collection on January 25, 2013, address: Wuhan University, Wuhan, deposit number is: CCTCC NO: M 2013055) appropriate amount, inoculated on PDA solid slant medium at 28 Cultivate in an incubator for 4 days.
[0018] Get the Trichoderma auranthoderma ( Trichoderma citrinoviride. ) appropriate amount of IBPT-4, inoculated into 400mL culture medium [Culture medium composition (g / L): mannitol 20.0, yeast extract 3.0, maltose 20.0, monosodium glutamate 10.0, glucose 10.0, KH 2 PO 4 0.5 , MgSO 4 0.3, NaCl 6.0, fixed volume into 1000mL Erlenmeyer flask], after static culture at 28°C for 30 days, mycelia and fermenta...
Embodiment 2
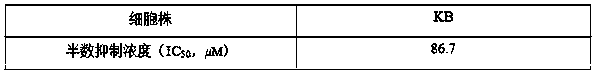
[0027] Example 2 Test of Anti-tumor Activity in Vitro
[0028] 1 Experimental samples and experimental methods
[0029] Preparation of the tested sample solution The test sample is the pure product of the compound separated and refined in the above-mentioned implementation 1. Accurately weigh an appropriate amount of sample, and prepare a solution with the required concentration with methanol for activity measurement.
[0030] Cell lines and cell subculture The oral epidermal carcinoma cell line was used, and the cells were subcultured in RPMI-1640 medium containing 10% FBS at 37°C in an incubator filled with 5% carbon dioxide.
[0031] Cell Proliferation Inhibitory Activity Test Method
[0032] Tetrazolium salt (MTT) method Take cells in the logarithmic growth phase and adjust the cell density to 2×10 per ml 5 Cells were inoculated into 96-well cell culture plates at 200 microliters per well, and filled with 5% CO at 37°C 2 for 4 hours in an incubator. Add 2 microliters ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com