A heptamethine near-infrared fluorescent molecular probe and its preparation method and application
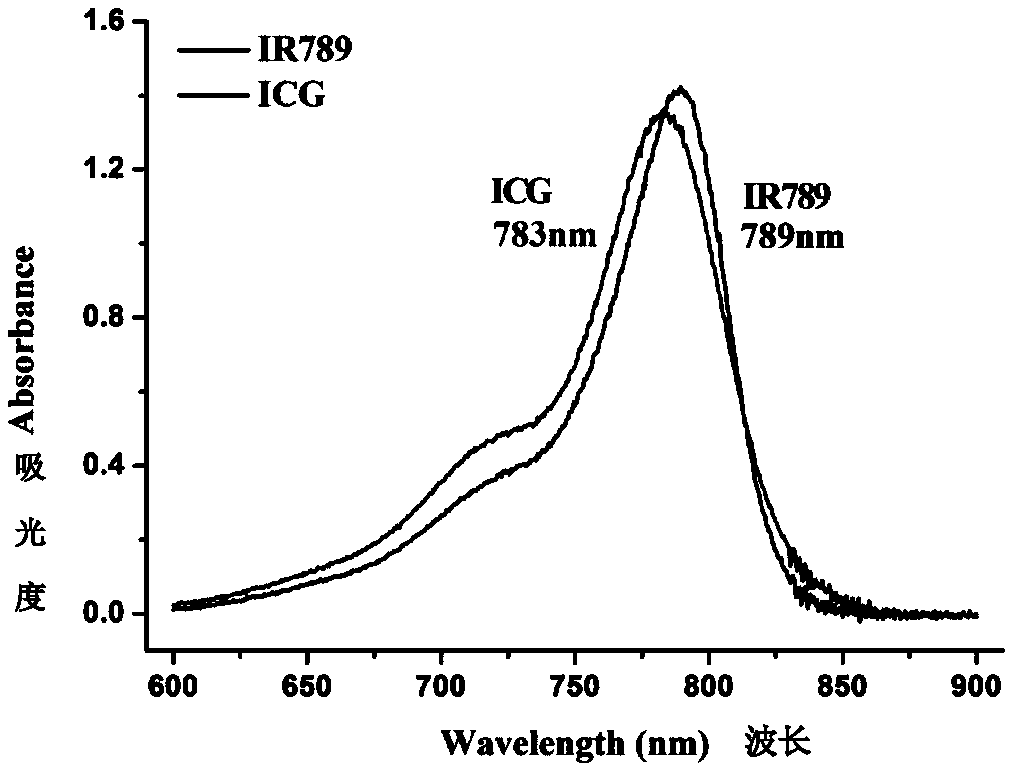
A technology of fluorescent molecular probe, heptamethine, which is applied in the preparation of the molecular probe and the application field of cell imaging, can solve problems such as instability, low quantum yield, leakage, etc., and achieve enhanced molecular fluorescence intensity, High sensitivity, increase the effect of stock displacement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
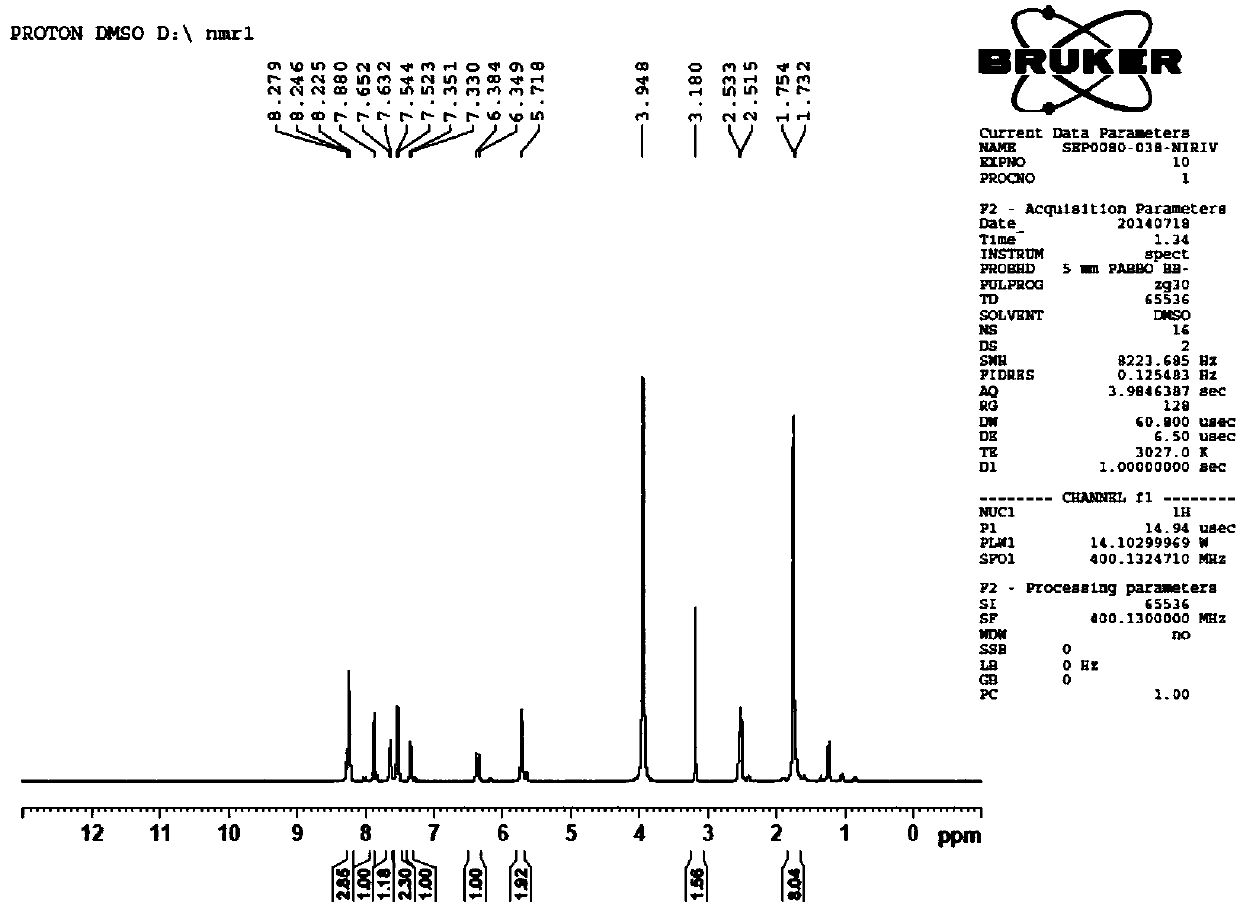
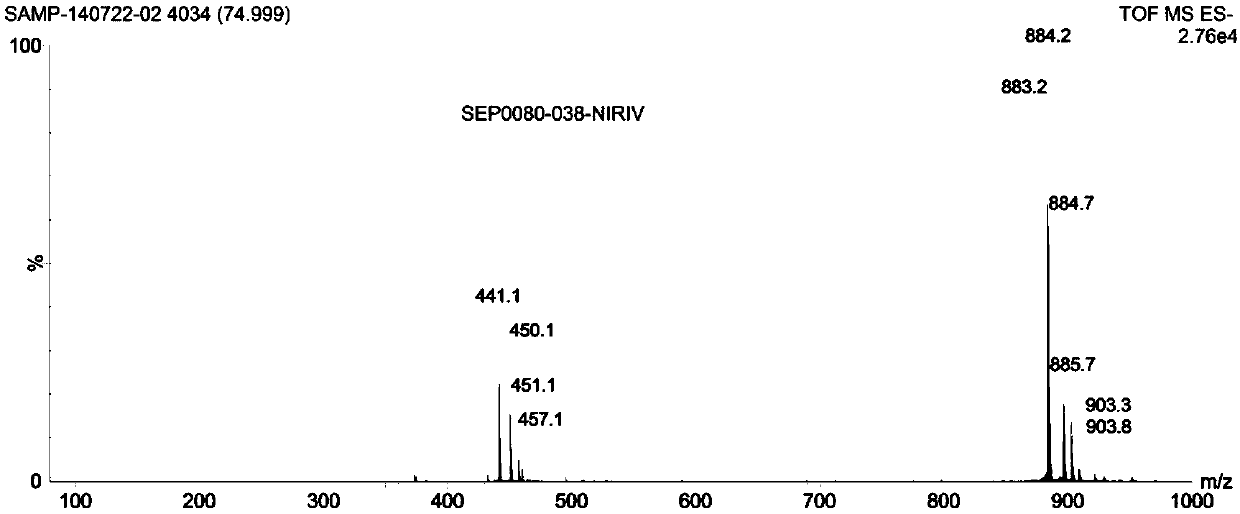
[0037] Add 3.21mmol of compound 1 (2,3,3-trimethyl-3H-indoline-5-sulfonate potassium), 4.17mmol of compound 2 (p-nitrobenzyl bromide) and 10ml of toluene in batches to a 50ml one-necked flask , under the protection of argon, reacted at 90°C for 16h, cooled to room temperature, filtered, washed with toluene, and dried in vacuo to obtain 0.94g brownish red solid (compound 3, 1-p-nitrobenzyl-2,3,3-tri Methyl-3H-indoline-5-sulfonic acid), yield 78.3%.
[0038] Take 1.98mmol of compound 3 and 0.99mmol of compound 4 (2-chloro-1-formyl-3-hydroxymethylenecyclohexene) into a 100ml reaction flask, add 30ml of n-butanol:toluene=7:3 In the mixed solvent, under the protection of argon, heat and reflux at 75°C for 6 hours. The solution turns from light red to deep red, and finally a large number of green components appear. After cooling down to room temperature, add 3 times the volume of the concentrated reaction solution after concentrating under reduced pressure. Diethyl ether was filter...
Embodiment 2
[0044] Add 3.21mmol of compound 1 (2,3,3-trimethyl-3H-indoline-5-sulfonate potassium), 8.34mmol of compound 2 (p-nitrobenzyl bromide) and 10ml of toluene in batches to a 50ml one-necked flask , under the protection of argon, reacted at 90 ° C for 15 h, cooled to room temperature, filtered, washed with toluene, and dried in vacuo to obtain 0.92 g of a brownish red solid (compound 3, 1-p-nitrobenzyl-2,3,3-tri methyl-3H-indoline-5-sulfonic acid).
[0045] Take 1.98mmol of compound 3 and 0.99mmol of compound 4 (2-chloro-1-formyl-3-hydroxymethylenecyclohexene) and add it to a 100ml reaction flask, add 30ml of absolute ethanol solvent, and then add 1.98mmol of Anhydrous sodium acetate was used as a catalyst, under the protection of argon, reflux reaction at 75°C for 4 hours, the reaction solution was brown, lowered to room temperature, concentrated under reduced pressure, added ether with 4 times the volume of the concentrated reaction solution, filtered, and used for filter cake Sep...
Embodiment 3
[0049] Add 3.21mmol compound 1 (2,3,3-trimethyl-3H-indoline-5-potassium sulfonate), 12.51mmol compound 2 (p-nitrobenzyl bromide) and 15ml toluene in batches to a 50ml one-necked flask , under the protection of argon, reacted at 85°C for 36h, cooled to room temperature, filtered, washed with toluene, and dried in vacuo to obtain 0.88g brownish red solid (compound 3, 1-p-nitrobenzyl-2,3,3-tri methyl-3H-indoline-5-sulfonic acid).
[0050] Get 1.98mmol of compound 3 and 0.99mmol of compound 4 (2-chloro-1-formyl-3-hydroxymethylenecyclohexene) and join in a 100ml reaction flask, add 30ml of pyridine as a reaction solvent, under the protection of argon reflux at 75°C for 8 hours, lower to room temperature, concentrate under reduced pressure, add 5 times the volume of diethyl ether of the concentrated reaction solution, filter, and separate the filter cake by column chromatography (silica gel column, the specification of silica gel is 200-300 mesh) For purification, the eluent was a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com