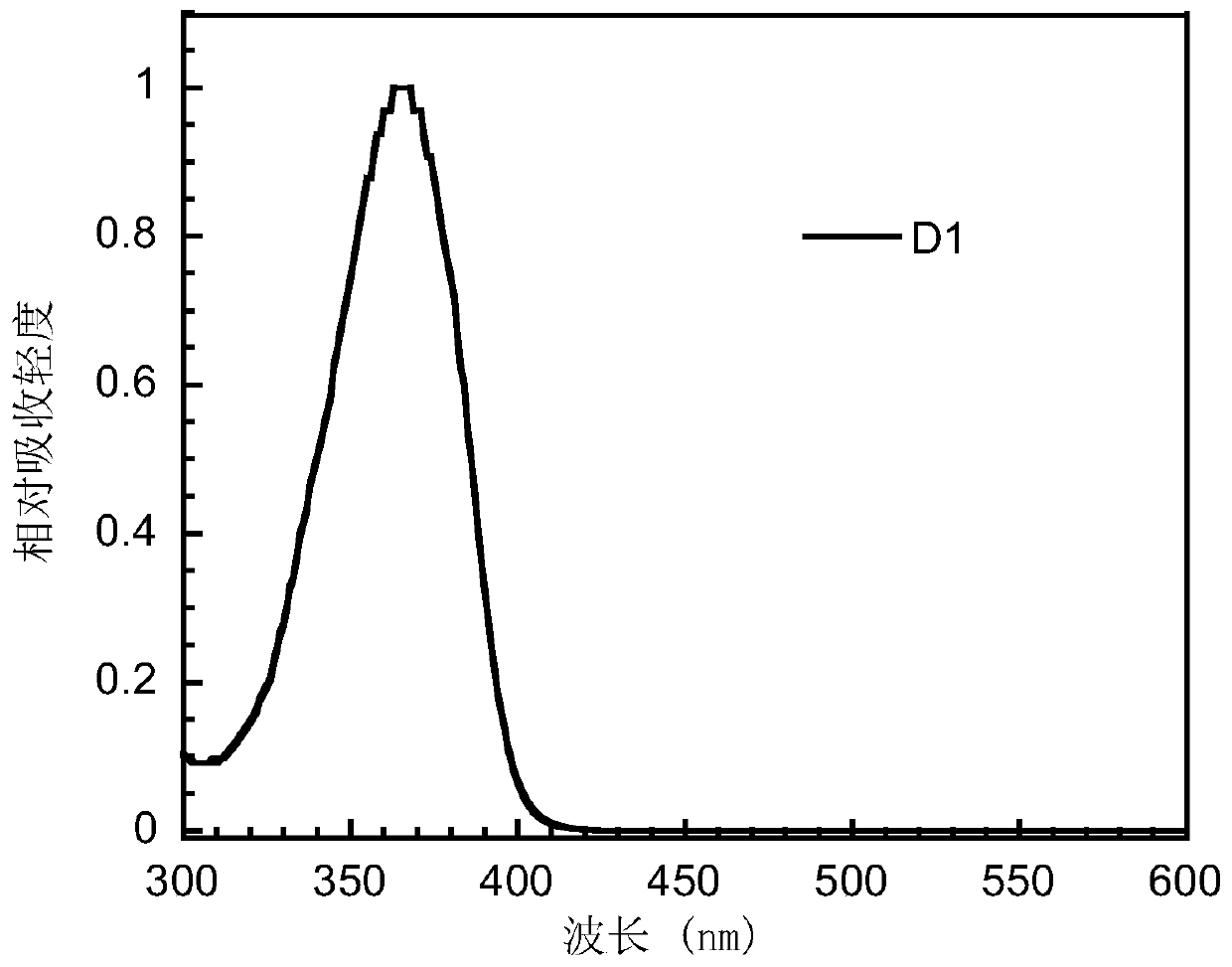
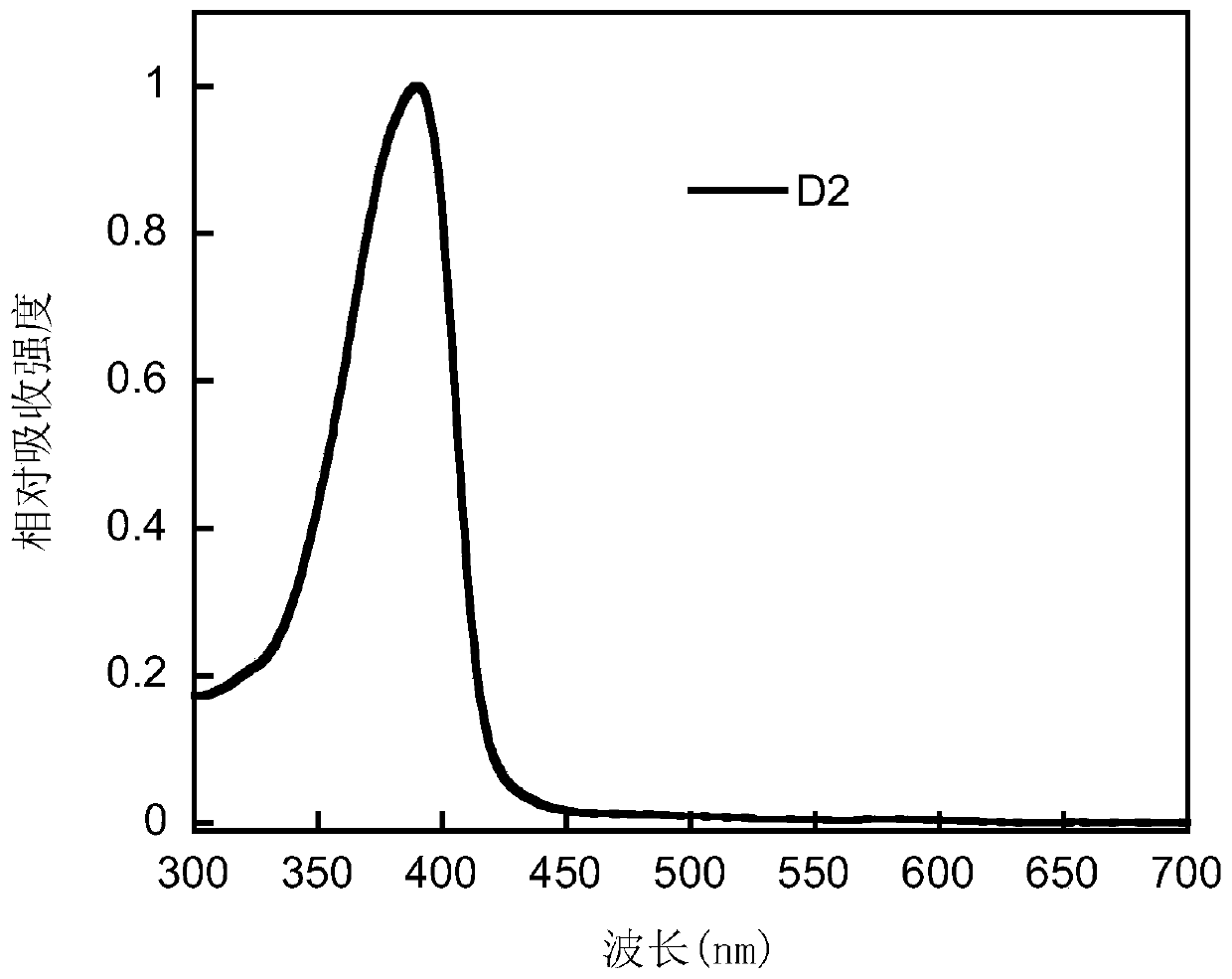
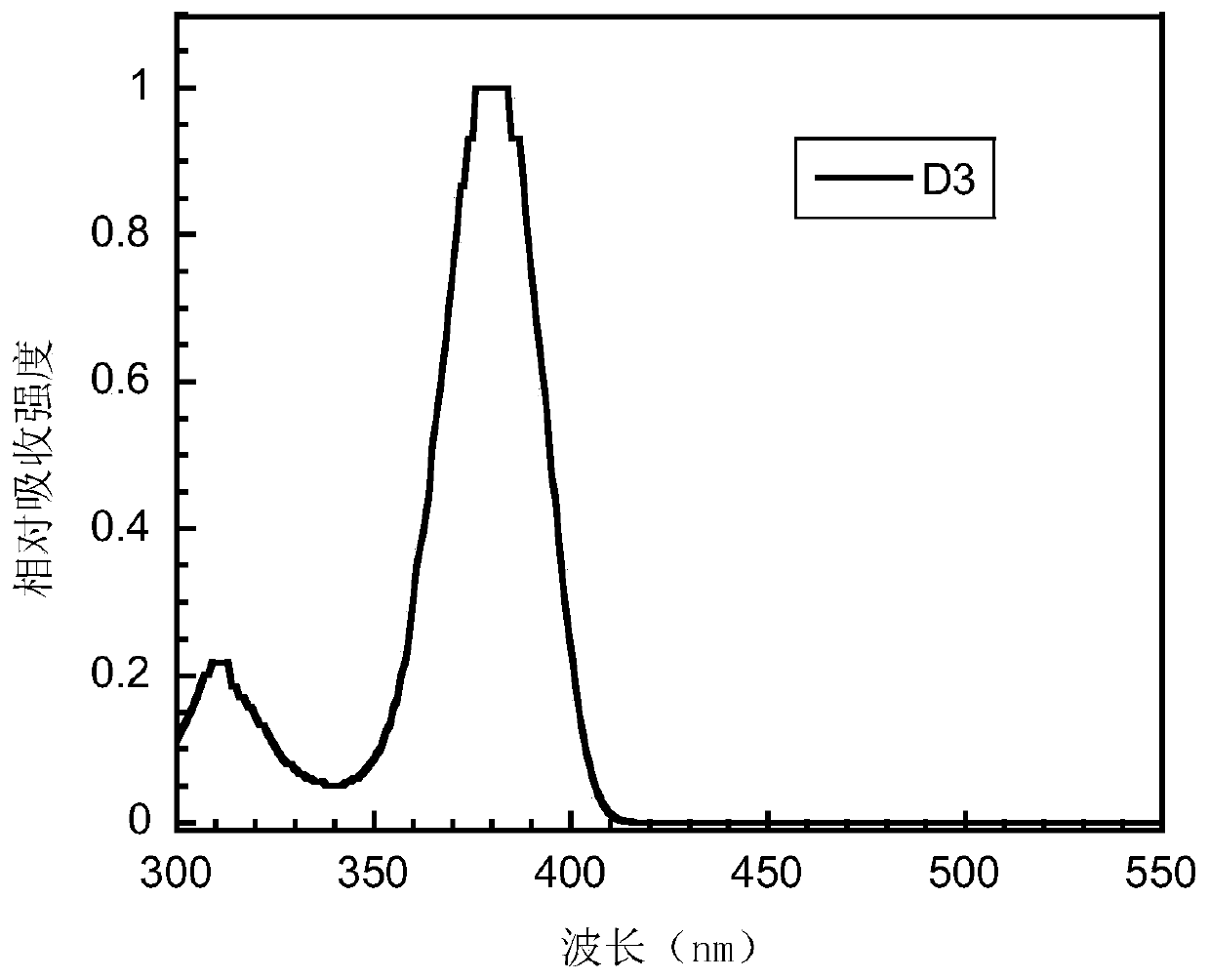
Bipolar small molecule luminescent material based on aromatic heterocyclo-2-s,s-dioxodibenzothiophene unit and its preparation method and application
A technology of dioxydibenzothiophene and -2-S, which is applied in the field of bipolar small molecule light-emitting materials and its preparation, achieves the effects of simple preparation process, balanced injection and transmission, and improved efficiency of materials and devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Methyl 1-bromo-2-quinoxalinecarboxylate
[0035] Under an argon atmosphere, add 1-bromo-2-quinoxalinecarboxylic acid (10g, 39.83mmol) into a two-necked flask, add 100mL of methanol, then add concentrated sulfuric acid (39.06mg, 398.29umol) dropwise, and heat to 110 °C, reacted for 18h. The reaction mixture was poured into water, extracted with ethyl acetate, and the organic layer was washed with brine and dried over anhydrous magnesium sulfate. After the solution was concentrated, a white solid crude product was obtained, which was purified by silica gel column chromatography (petroleum ether / dichloromethane=3 / 1, v / v was selected as the eluent), and the product was placed in the refrigerator for a long time to obtain a white solid with a yield of 85%. . 1 H NMR, 13 CNMR, MS and elemental analysis results show that the compound obtained is the target product, and its chemical reaction equation is as follows:
[0036]
Embodiment 2
[0038] Preparation of 2-bromothiofluorene
[0039] Under an argon atmosphere, add thiofluorene (20g, 108.54mmol) into a 250ml two-necked bottle, then add 100ml of chloroform to dissolve completely, add iodine (275.39mg, 1.09mmol), drop by drop under the condition of avoiding light Add liquid bromine (38.16g, 108.54mmol), the reaction solution was stirred under ice bath for 2 hours, then stirred at room temperature for 2 hours, adding saturated sodium bisulfite to quench the liquid bromine, the reaction mixture was poured into water, washed with acetic acid Extracted with ethyl ester, the organic layer was washed completely with brine, and dried over anhydrous magnesium sulfate. After the solution was concentrated, a crude white solid was obtained, which was then recrystallized from chloroform with a yield of 85%. 1 HNMR, 13CNMR, MS and elemental analysis results show that the obtained compound is the target product, and its chemical reaction equation is as follows:
[0040]...
Embodiment 3
[0042] 2-boronate thiofluorene
[0043] Under an argon atmosphere, 2-bromothiofluorene (10 g, 29.24 mmol) was dissolved in 180 mL of refined tetrahydrofuran (THF), and 1.6 mol L of -1 28mL of n-butyllithium, reacted for 2 hours, then quickly added 25mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborane, at -78℃ The reaction was continued for 1 hour, and the temperature was slowly raised to room temperature for 24 hours. The reaction mixture was poured into water, extracted with ethyl acetate, and the organic layer was washed with brine and dried over anhydrous magnesium sulfate. After the solution was concentrated, a light yellow viscous crude product was obtained, which was purified by silica gel column chromatography (petroleum ether / ethyl acetate=20 / 1, v / v was selected as the eluent), and the product was placed in the refrigerator for a long time to obtain a white solid, the product rate of 70%. 1 H NMR and GC-MASS tests showed that it was the target product. Its che...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com