An organic electroluminescent device based on 10,10-diaryl anthrone compound and its application
An electroluminescent device, diaryl anthrone technology, applied in the field of semiconductors, can solve difficult problems such as high exciton utilization rate and high fluorescence radiation efficiency, low S1 state radiation transition rate, efficiency roll-off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
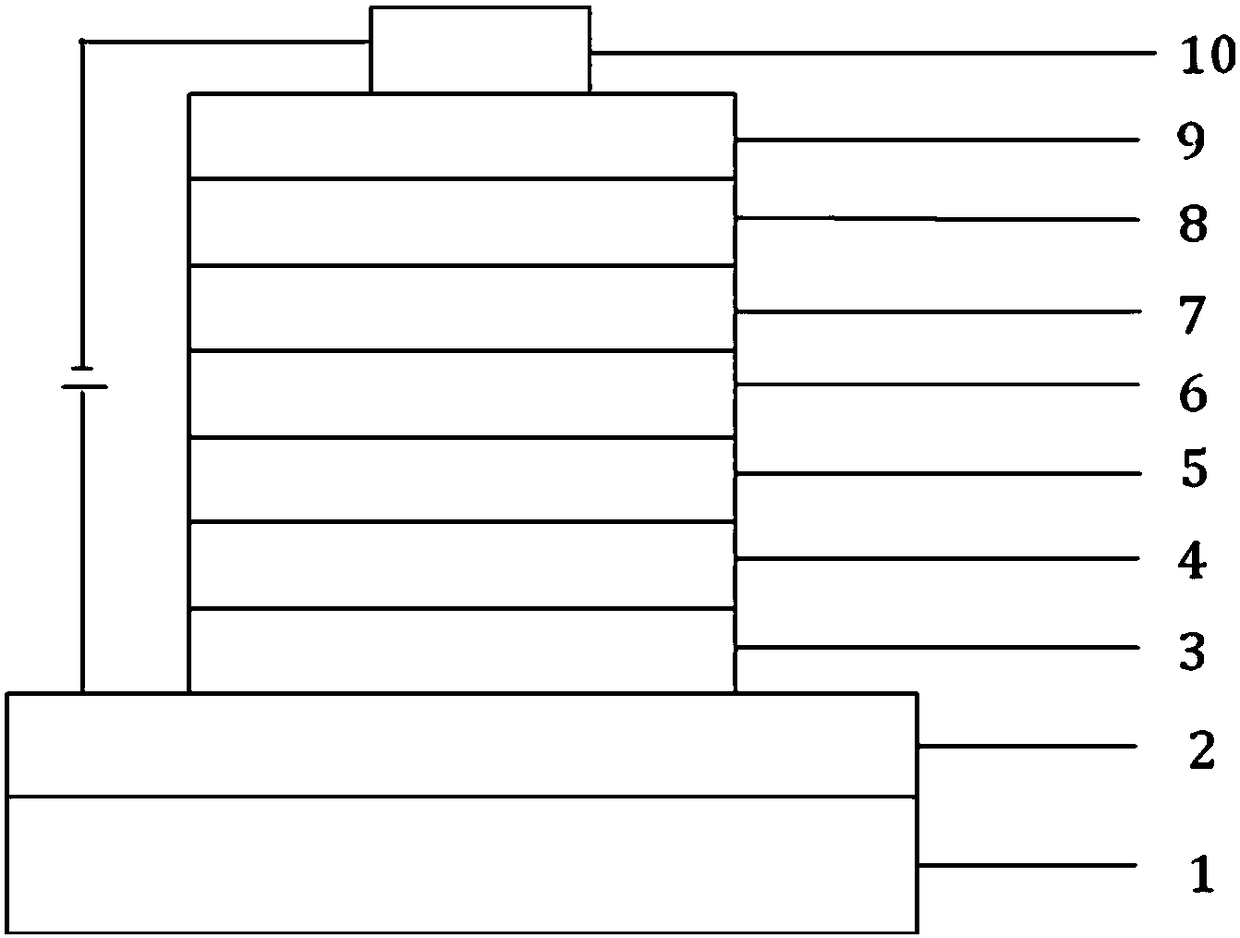
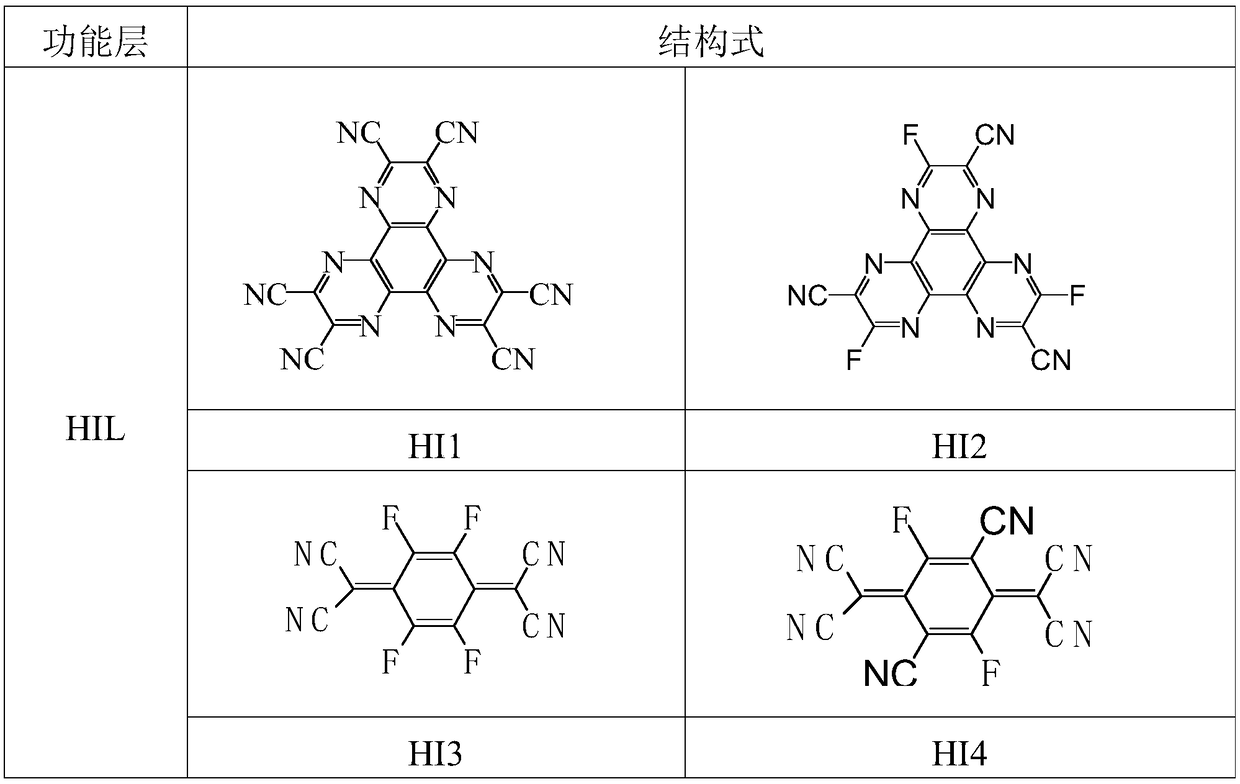
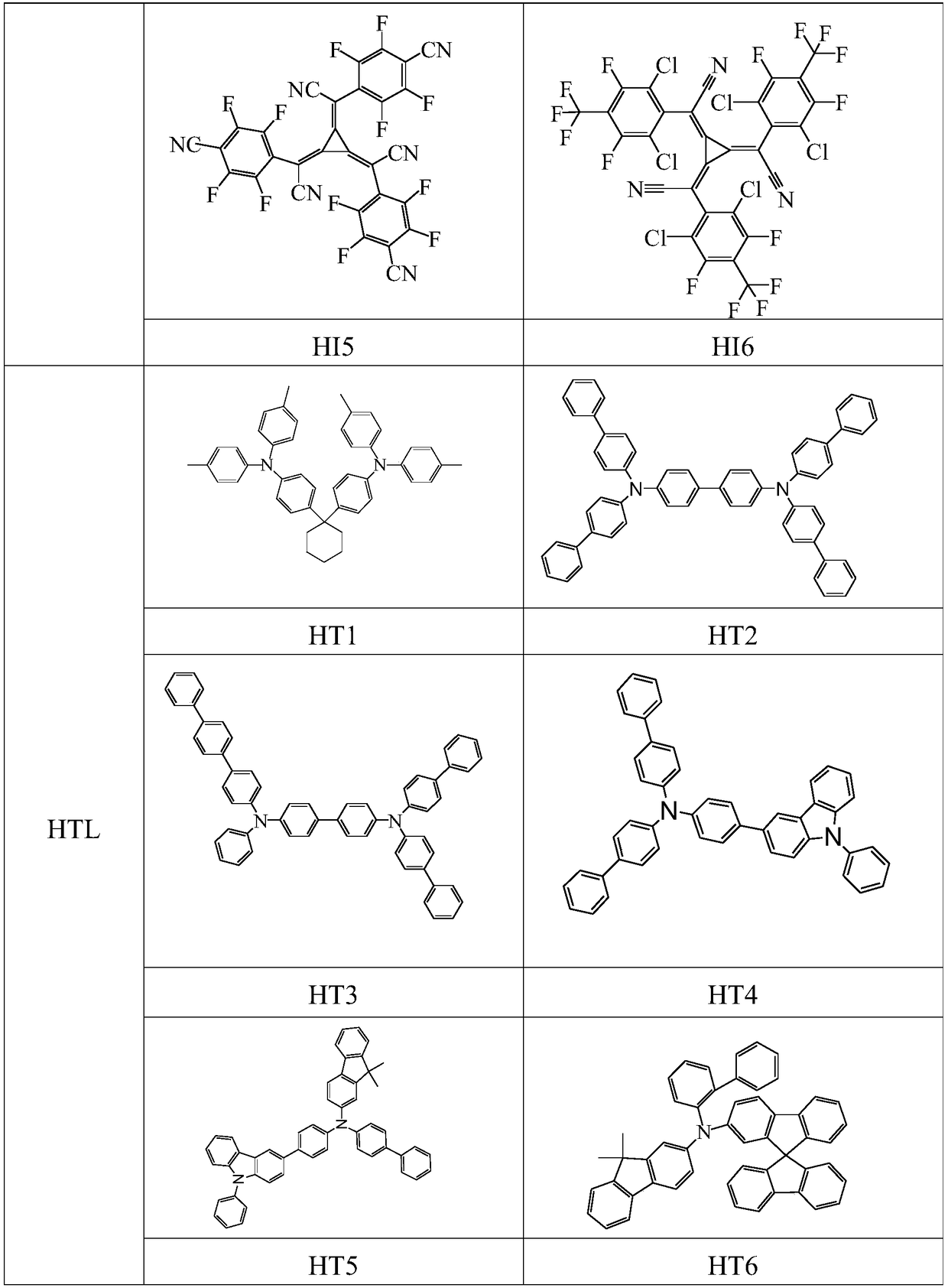
Image
Examples
Embodiment 1
[0067] Synthesis of Example 1 Raw Material A
[0068]
[0069] In a 1L three-necked flask, add 19.8g phenol (0.21mol), 20.8g anthraquinone (0.10mol), 0.2g mercaptopropionic acid, 400mL dichloroethane, mix and stir, heat up to 60~65 ℃ and add dropwise 3.0g methyl alcohol The sulfonic acid was added dropwise, and the reaction was incubated for 4 hours at 60 to 65 ° C; after the reaction was completed, the temperature was cooled, washed with water, and the organic phase was decompressed to remove the solvent to obtain a crude product, which was recrystallized with absolute ethanol and obtained by column chromatography. White crystal - compound X, HPLC purity 99.5%, yield 46.58%;
[0070] 18.9g of compound X (0.05mol) and 100g of pyridine were added to a 500mL three-necked flask, the temperature was lowered to 0~5°C and 33.8g of trifluoromethanesulfonic anhydride (0.12mol) was added dropwise, and the reaction was carried out at room temperature for 6 hours; The solvent was rem...
Embodiment 2
[0073] The synthesis of embodiment 2 raw material B
[0074]
[0075] In a 250ml three-necked flask, under nitrogen protection, add 6.42g raw material A (0.01mol), 10.16g bis-borate pinacol ester (0.04mol), 4.90g potassium acetate (0.05mol), 0.30g pd2(dba)3 , 0.20g tri-tert-butylphosphorus, 100ml toluene, refluxed for 20 hours; after the reaction, cooling, filtering, rotary evaporation of the filtrate, column chromatography to obtain raw material B, HPLC purity 99.8%, yield 88.26%;
[0076] High resolution mass spectrometry ESI source, positive ion mode, molecular formula C38H40B2O5, theoretical value: 598.3062, tested value: 598.3066.
[0077] Elemental analysis (C38H40B2O5): Theoretical C: 76.28, H: 6.74, O: 13.37, Tested C: 76.26, H: 6.75, O: 13.40.
Embodiment 3
[0078] Example 3 Synthesis of Compound 1
[0079] synthetic route:
[0080]
[0081] In a 250ml three-necked flask, under nitrogen protection, add 3.21g raw material A (0.005mol), 2.20g compound M01 (0.012mol), 1.44g sodium tert-butoxide (0.015mol), 0.15g pd2(dba)3, 0.10g Tri-tert-butylphosphorus, 100 ml of toluene, refluxed for 20 hours; after the reaction, cooling, filtration, rotary evaporation of the filtrate, and column chromatography to obtain compound 1, HPLC purity 99.9%, yield 78.40%;
[0082] High resolution mass spectrometry ESI source, positive ion mode, molecular formula C50H32N2O3, theoretical value: 708.2413, test value: 708.2411.
[0083] Elemental analysis (C50H32N2O3): Theoretical C: 84.73, H: 4.55, N: 3.95, O: 6.77, Tested C: 84.71, H: 4.55, N: 3.98, O: 6.76.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com