Methods of treating huntington's disease using cysteamine compositions
A technology for Huntington's disease and cysteamine, applied in drug combinations, pharmaceutical formulations, nervous system diseases, etc., can solve problems such as inability to improve cognition and slow decline in motor function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0143] Clinical Trial Protocol
[0144] Research institute
[0145] In this double-blind, placebo-controlled, multicenter trial, the efficacy, safety, and tolerability of enteric-coated cysteamine in modulating the progression of HD as measured by the Unified Huntington's Disease Rating Scale ( Changes in the Total Motor Score (TMS) of the UHCRS). Patients were recruited from nine departments of neurology and genetics throughout France. Protocol and informed consent were approved by an institutional review board according to French law.
[0146] participant
[0147] Male and female HD patients aged 18 to 65 with CAG repeats >38 in the HTT gene were recruited. Inclusion criteria were minimum scores on both components of the Unified Huntington's Disease Rating Scale (UHDRS) (18): Total Motor Score (TMS) >5 and Total Functional Capacity (TFC) >10. The UHDRS is a validated scale for assessing clinical performance and abilities in four domains: motor function, cognitive function...
example 2
[0163] Twice-daily cysteamine improves motor scores in HD patients
[0164] From October 2010 to June 2012, a total of 96 subjects were randomized to treatment and constituted the ITT cohort. The baseline characteristics of the ITT and NoTBZ populations are shown in Table 1.
[0165] Table 1:
[0166]
[0167]
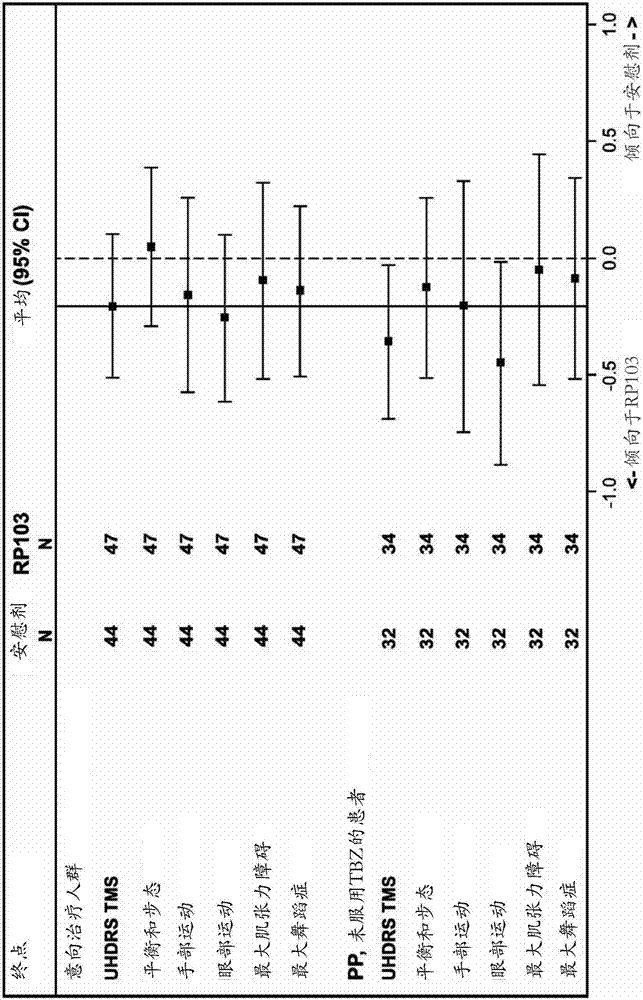
[0168] Efficacy on TMS
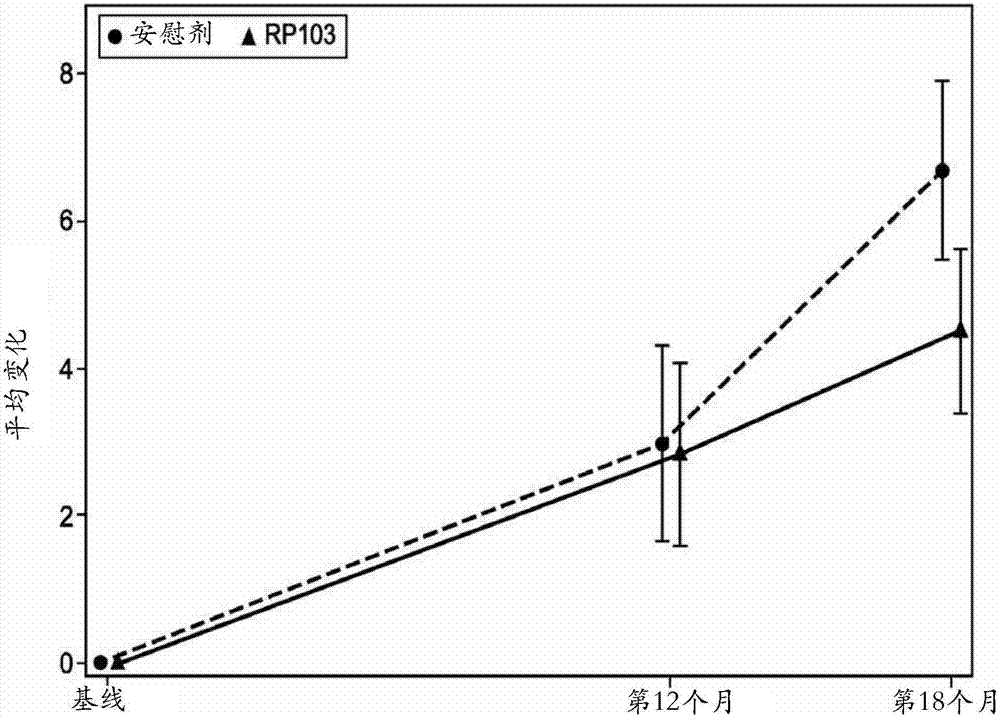
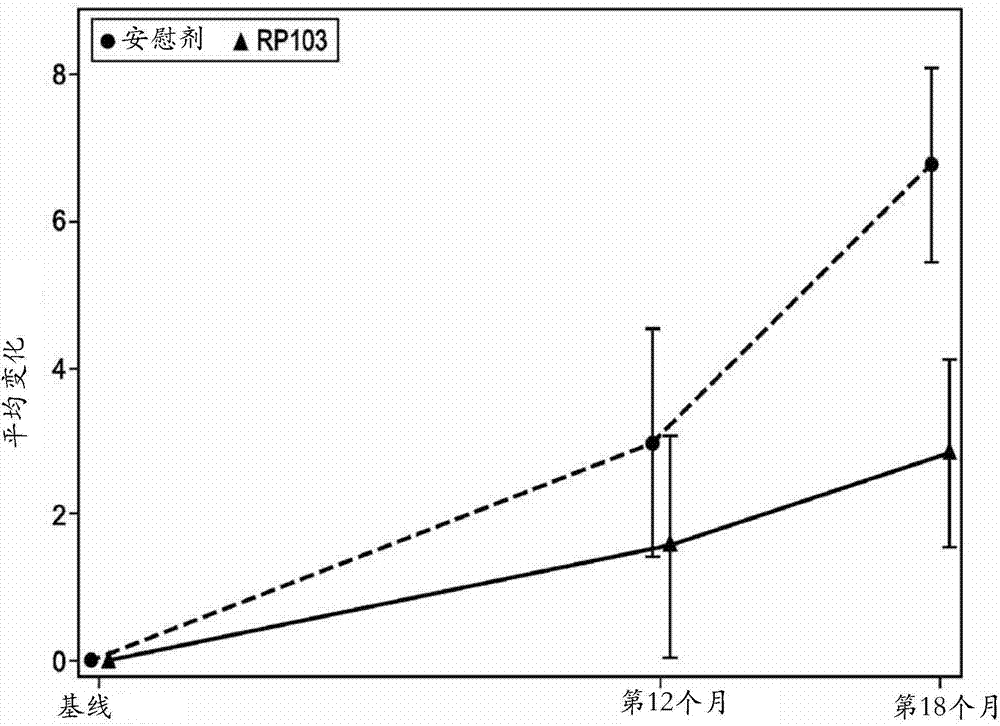
[0169] The ITT analysis of all 96 patients enrolled in the trial showed a slow positive trend toward progression to TMS (the primary endpoint of the study) for patients who received placebo and those who received RP103. The change in the primary endpoint from baseline to 18 months in TMS was 6.68 ± 7.98 for placebo and 4.55 ± 8.24 for RP103. Through the primary analysis method, the difference between the groups was 1.593±1.709, which was not statistically significant (95%CI[-5.000; 1.815]; p=0.3545). Supportive analyzes showed a between-group difference of 2.33±1.72 (95% CI [-5.750; 1.085]; p=0.1785) in the ITT population and 2.20±1.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com