Metal organic liquid crystal dyes
A liquid crystal, metal technology, applied in the field of dye components, polymer dispersed liquid crystal, can solve the problem of uneven projection spectrum of metal organic mesogen composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Synthesis of HL2 Ligand: C22H28N2O
[0190] The synthesis of the HL2 ligand C22H28N2O was obtained by diazotization of 4-hexylaniline. Subsequent coupling with phenol affords 4-hydroxy-4'-hexylazobenzene (I) and etherification with 4-bromo-1-butene as shown in the following scheme:
[0191]
[0192] 8.5 mmol (1.5 g) of 4-hexylaniline were dissolved in 8 mL of water and 2.1 mL of HCl (36%) under nitrogen. The solution was cooled at 0°C, and 8 mL of aqueous NaNO2 (0.6 g, 9.1 mmol) was added, keeping the solution temperature below 5°C. Phenol (0.8 g, 8.5 mmol) dissolved in 2.5 mL NaOH 2N was added dropwise to the solution of the diazonium salt. The reaction mixture was warmed to room temperature and extracted with dichloromethane; the organic layer was dried over anhydrous sodium sulfate and evaporated under reduced pressure. The crude product I was purified by chromatography (silica gel, hexane / ether, 1 / 1) and recrystallized from n-heptane to give the pure product a...
Embodiment 2
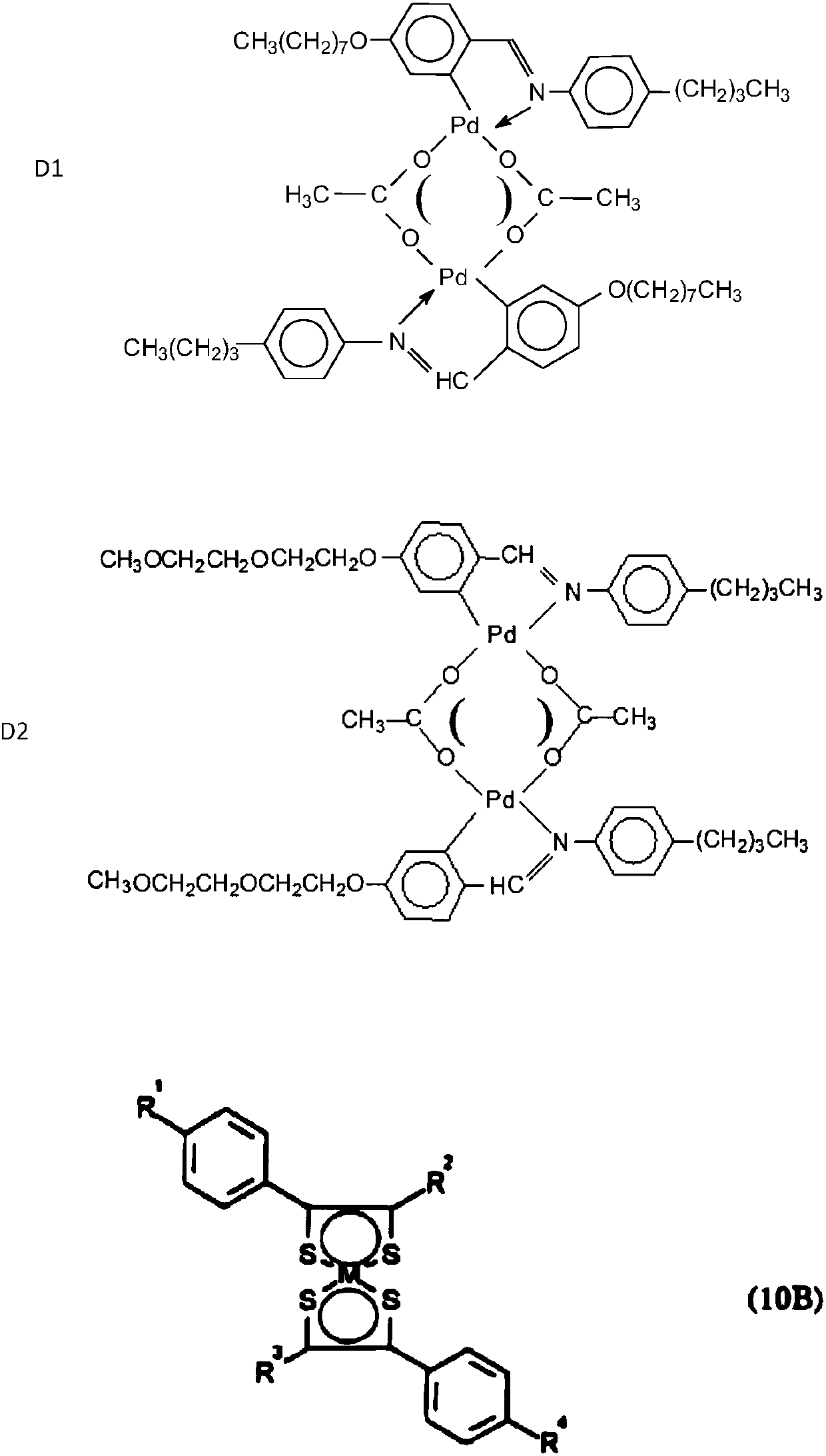
[0203] Synthesis of Metal Mesogenic Complex L2Pd-acac(C27H34N2O3-Pd)
[0204] The synthesis of metal-mesomorphic (MOM) C27H34N2O3-Pd complex is accomplished through the following two-step reaction:
[0205]
[0206] First, the ring-palladiumation of the ligand 4-(3-butenyloxy)-4'-hexylazobenzene 4 with [(PhCN)2PdC12] gave the dinuclear chlorine-bridged derivative [LPd(μ-Cl) ]2, then obtained as a mixture of isomers of A and B complexes by a bridge-splitting reaction with ethyl-pyruvate potassium complex 5 in a molar ratio of 2:1, composed of two Obtained by non-selective precipitation of the benzene ring. To a suspension of 0.1 g of 4 in methanol (5 mL) was added an equimolar amount of [Pd(PhCN)Cl]2 (0.11 g) dissolved in 6 mL of benzene. The mixture was stirred at room temperature for 24 h and the brown solid formed was filtered off and recrystallized from dichloromethane / ethanol to give the pure product [LPd(μ-Cl)]2 as an ocher yellow solid in 83% yield ( 0.12G). In a ...
Embodiment -3
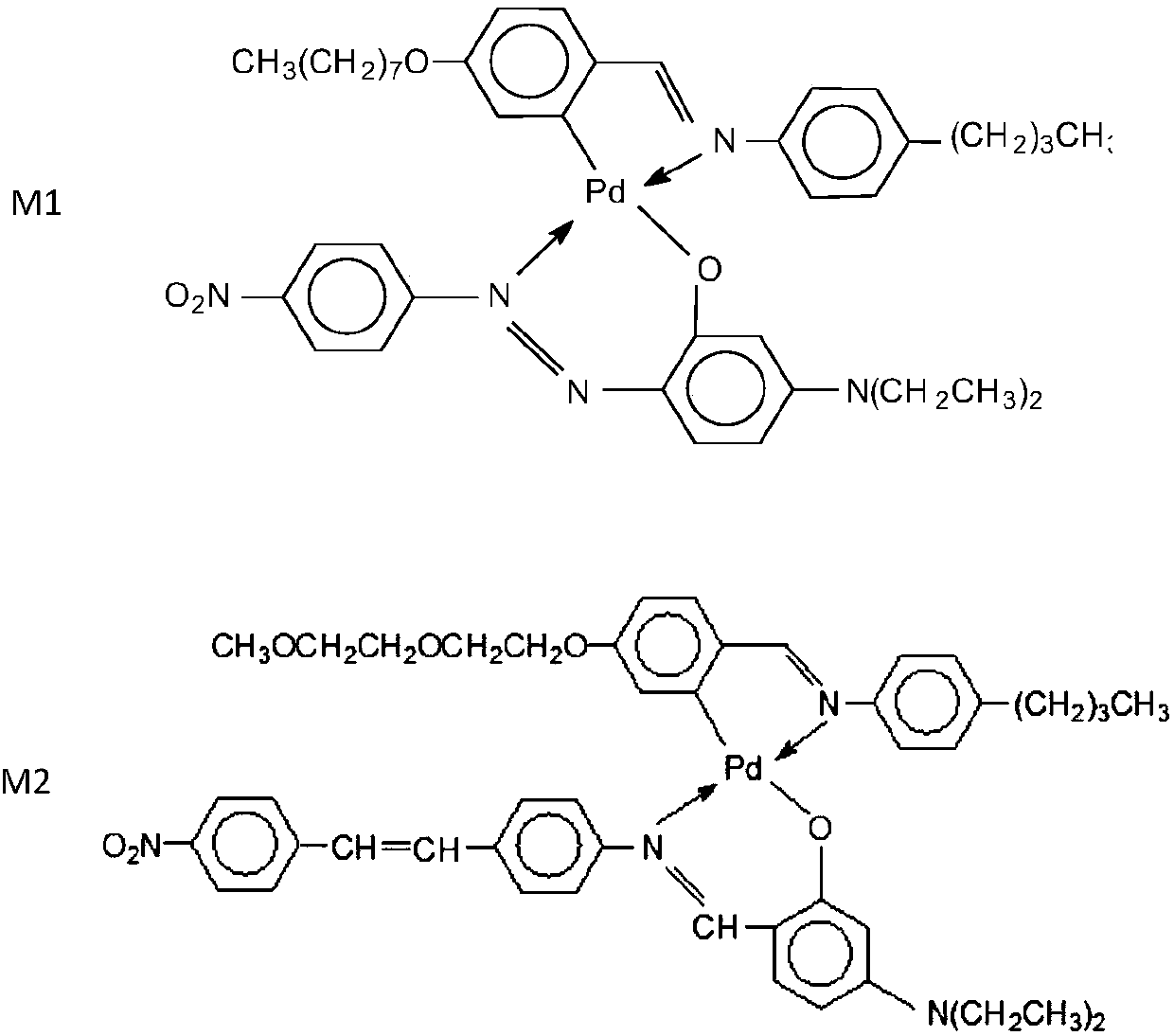
[0218] Synthesis of a one-pot chemical mixture of salicylic acid diamine metal mesogens
[0219] Complex
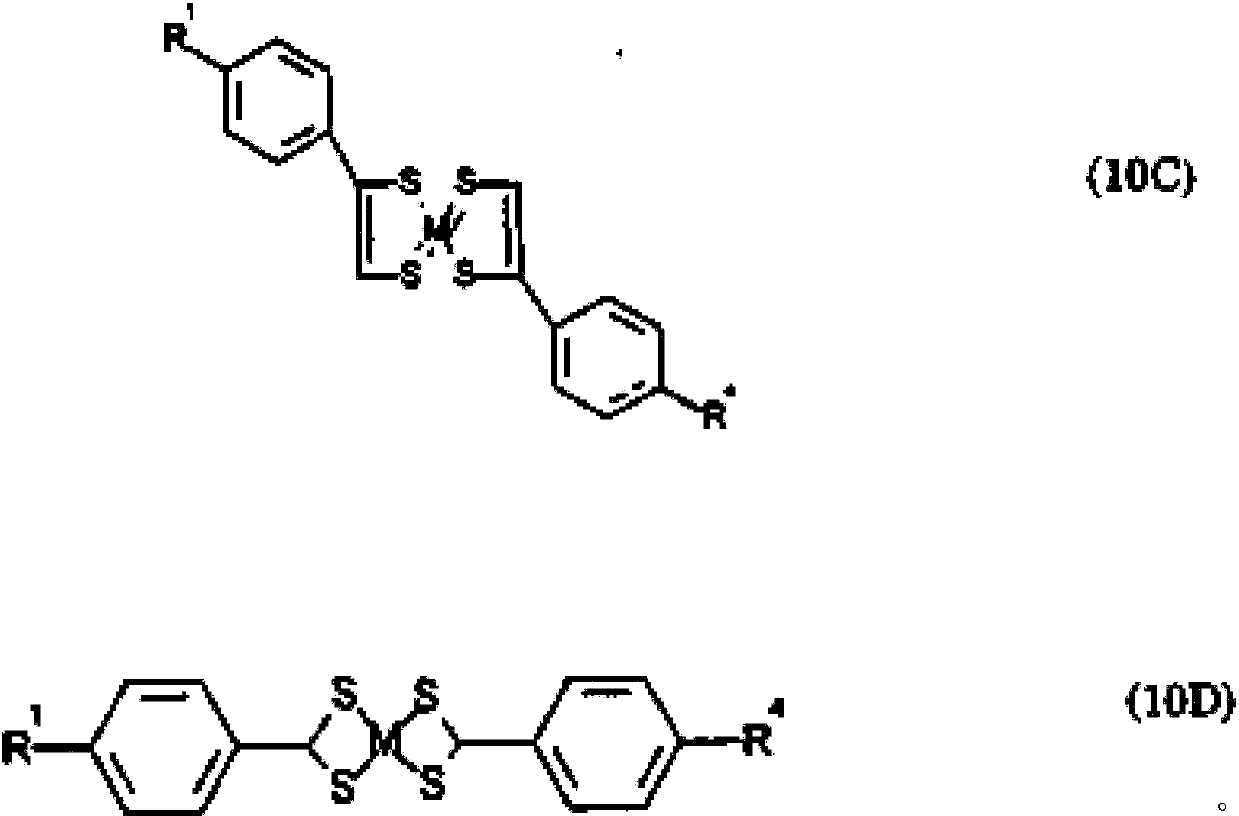
[0220] "One-pot" solution synthesis: The synthesis of "one-pot" multi-component metal mesomorphic mixtures based on salicylic acid diamine metal complexes proceeds by the simultaneous reaction of suitable precursors, as in the single-component metal Synthesis in mesogens. Each "in situ" mixture consists of a three-component system corresponding to the following general chemical structure:
[0221]
[0222] Two heterofunctional linkage (two aldehydes) molecules react simultaneously with amines and finally with metal ions. Although only two heterofunctional groups (aldehydes and / or amines) are used, simultaneous synthesis of more than two heterofunctional species can provide an increasing number of structurally distinct metal complexes. Six three-component "in situ" metallo-mesomorphic mixtures were prepared under various combinations of metals and ligands. Each comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com