A kind of preparation method of p-cyanobenzoic acid
A technology of cyanobenzoic acid and chloromethyl benzonitrile is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, chemical instruments and methods, etc. problems, to achieve the effect of improving product purity and yield, simple reaction steps, and low reaction energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
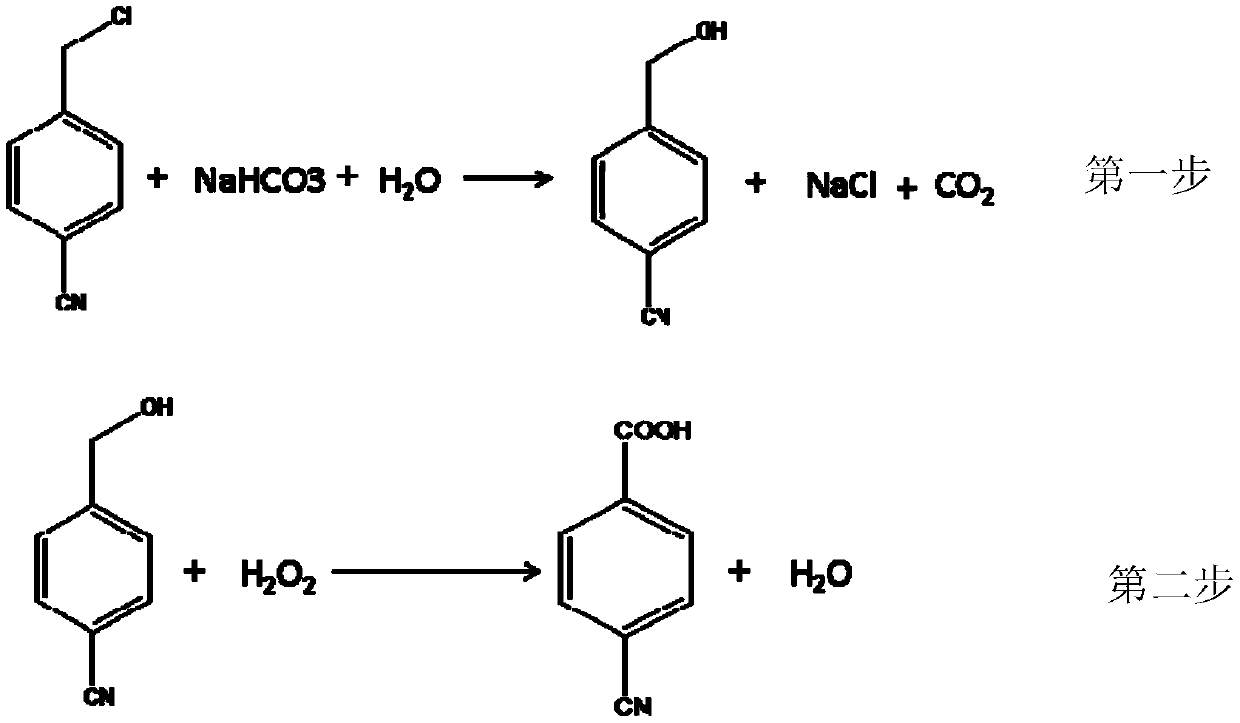
[0032] Put 138g of potassium carbonate, 151.5g of 4-chloromethylbenzonitrile and 2300g of water into a 3000mL four-necked bottle. Heat to 80°C, react for 10 hours, take a sample for HPLC detection, confirm that the hydrolysis reaction of 4-chloromethylbenzonitrile is complete, add dichloromethane to the hydrolysis reaction solution and extract (each time 800mL, extract 3 times at room temperature) , the extract was distilled off to remove the extraction solvent to obtain 128 g of 4-cyanobenzyl alcohol with a purity of 96-98% and a yield of 96.2%.
[0033] Put 128g of 4-cyanobenzyl alcohol, 6.2g of methyl trioctyl ammonium bisulfate, 4.6g of sodium tungstate dihydrate, 4g of vanadyl sulfate, 4g of sodium chloride, and 500g of water into a four-necked bottle. Heated the bath to raise the temperature to 90°C, and added 400 g of 30% hydrogen peroxide dropwise to the kettle. Sampling was carried out for HPLC detection. When 4-cyanobenzyl alcohol disappeared, the reaction was compl...
Embodiment 2
[0035] Add 10g of potassium bicarbonate, 15.1g of 4-chloromethylbenzonitrile and 150g of water into a 250mL four-necked bottle, raise the temperature to 80°C under full stirring, and react for 10 hours. After HPLC detection, it is confirmed that the hydrolysis reaction of the raw materials is complete, and the reaction Chloroform was added to the solution for partial extraction (100 mL each time, extracted 3 times at room temperature), and the extract was distilled to remove the extraction solvent to obtain a total of 12.6 g of intermediate 4-cyanobenzyl alcohol, with a purity of 93%-95%. was 94.7%.
[0036] Put 12.6g of 4-cyanobenzyl alcohol, 0.62g of methyl trioctyl ammonium chloride, 0.46g of sodium tungstate dihydrate, 0.4g of sodium sulfate, 0.4g of vanadyl sulfate, tert-butanol into a 500mL four-necked bottle 50g, raise the temperature of the kettle to 80°C, slowly add 200g of tert-butyl hydroperoxide dropwise with a micro-dropping funnel, and perform HPLC detection by s...
Embodiment 3
[0038] Add 10 g of potassium hydroxide, 15.1 g of 4-chloromethylbenzonitrile and 120 g of water into a 250 mL four-necked bottle to carry out the hydrolysis reaction of the raw materials. Under mechanical stirring, it was heated to reflux (82° C.), and detected by HPLC, it can be seen that the raw materials had been completely reacted after 10 hours of reaction. At this point, petroleum ether was added for partial extraction (80 mL each time, extracted 3 times at normal temperature), and the extract was distilled to remove the extraction solvent to obtain the intermediate 4-cyanobenzyl alcohol, a total of 11.4 g, with a purity of 88-92%. The rate is 85.7%.
[0039] In a 250mL four-necked bottle, add 11.4g of 4-cyanobenzyl alcohol, 0.62g of tetrabutylammonium chloride, 0.46g of sodium tungstate, 0.4g of sodium sulfate, 0.4g of vanadyl sulfate, 60g of sodium hypochlorite, and 50g of ethanol. The temperature was raised to 78°C, and HPLC detection was carried out by sampling. Whe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com