Electrochemical study method closer to real mineral floatation
A mineral flotation and electrochemical technology, applied in the direction of material electrochemical variables, scientific instruments, material analysis through electromagnetic means, etc., can solve problems that cannot fully represent the real flotation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Making mineral electrodes:
[0019] Cut the massive pyrite into a square column with a length, width and height of 3*3*15mm, and bond a copper wire to the bottom of the columnar pyrite with silver conductive adhesive; it is made of thin aluminum sheet A cylinder with a diameter of 6mm and a height of 15mm, then put the columnar pyrite bonded with the copper wire into the aluminum cylinder, fill the gap in the aluminum cylinder with epoxy resin, and remove it after the epoxy resin is completely cured The aluminum cylinder of the outer layer, promptly makes the spare mineral electrode;
[0020] The filling height of the epoxy resin in the A electrode of the mineral electrode is 3mm, and the exposed height of the columnar pyrite is 12mm; the filling height of the epoxy resin in the B electrode is 15mm, and the exposed height of the columnar pyrite is 0mm;
[0021] (2) Configure the electrolyte:
[0022] Configure a buffer solution with a pH value of 5: Take 13.6 gram...
Embodiment 2
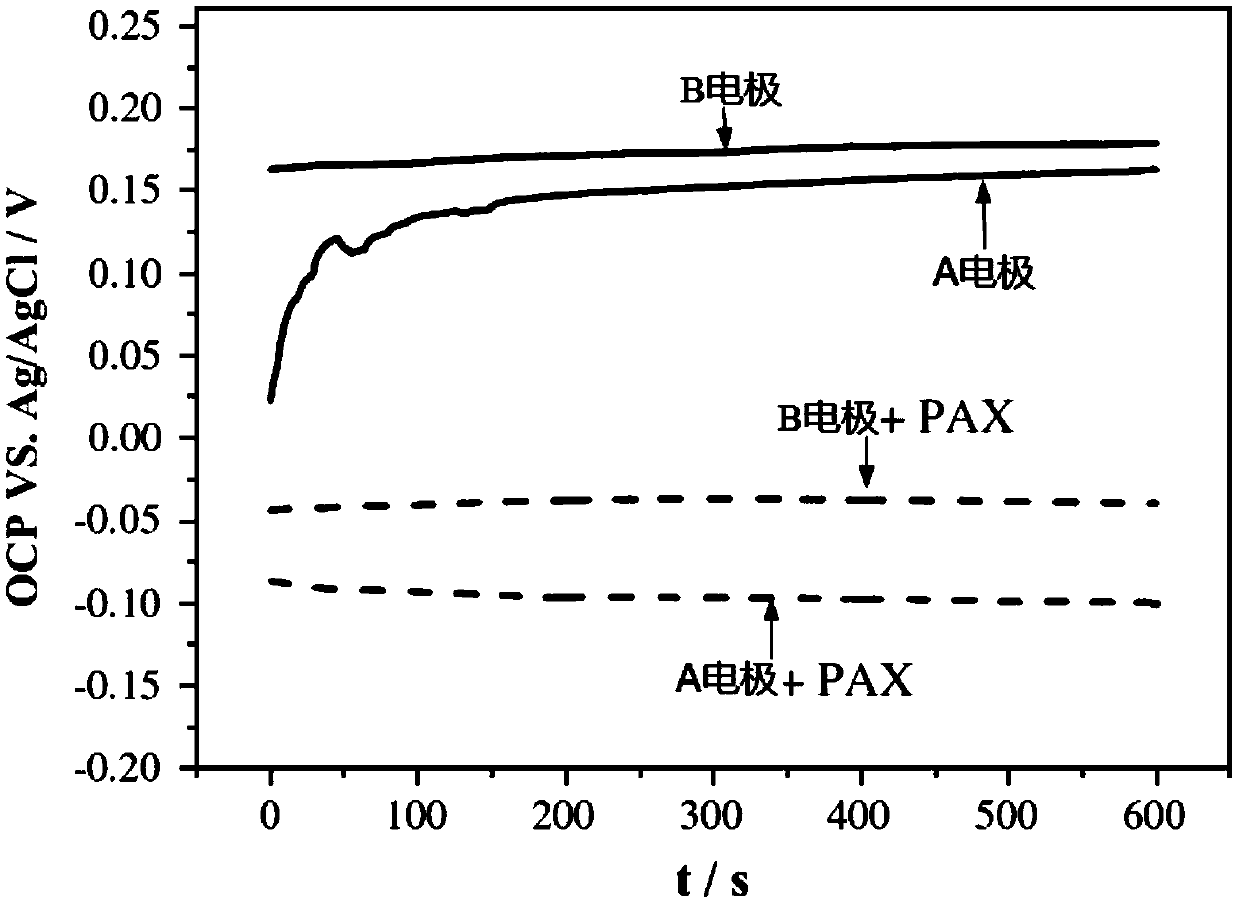
[0030] The steps are the same as in Example 1, except that the size of the massive pyrite cut is changed from "3*3*15mm" to "5*5*20mm", and other conditions remain unchanged. The results are as follows: OCP was tested in a buffer solution with a pH value of 5. When no xanthate was added, the OCP of the B electrode was always in a stable state at 0.17V from 0 to 600 seconds; the OCP of the A electrode started It is much lower than that of the B electrode, and then rises rapidly until it gradually stabilizes after 410 seconds, which is 0.16V. When xanthate was added, the OCP of both A and B electrodes was greatly reduced, and the OCP value of A electrode (-0.08) was lower than that of B electrode (-0.03).
Embodiment 3
[0032] The steps are the same as in Example 1, the OCP experiment time is changed from "0-600 seconds" to "0-400 seconds", and other conditions are unchanged. The results are as follows: OCP was tested in a buffer solution with a pH value of 5. When no xanthate was added, the OCP of the B electrode was always in a stable state at 0.17V during the time from 0 to 400 seconds; the OCP of the A electrode started It is much lower than that of the B electrode, and then rises rapidly, and it is 0.14V in 400 seconds. When xanthate was added, the OCP of both A and B electrodes was greatly reduced, and the OCP value of A electrode (-0.07) was lower than that of B electrode (-0.03).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap