New uses of naphthalene ring drugs
A pharmacy and compound technology, applied in the field of amide drugs and their application in inhibiting transient receptor potential channel proteins, can solve problems such as unclear domain functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
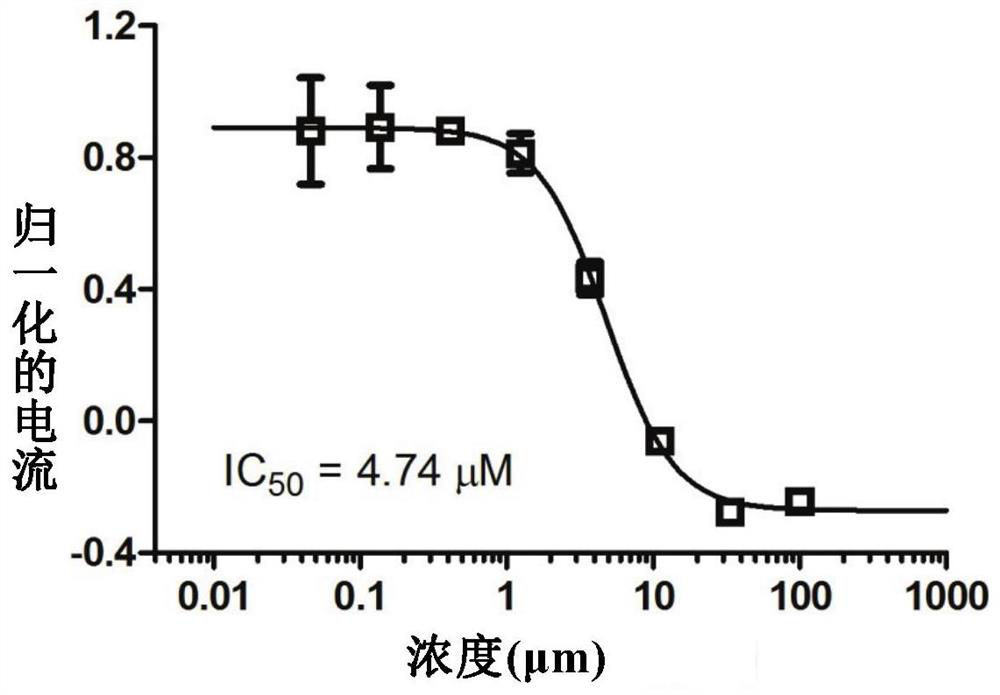
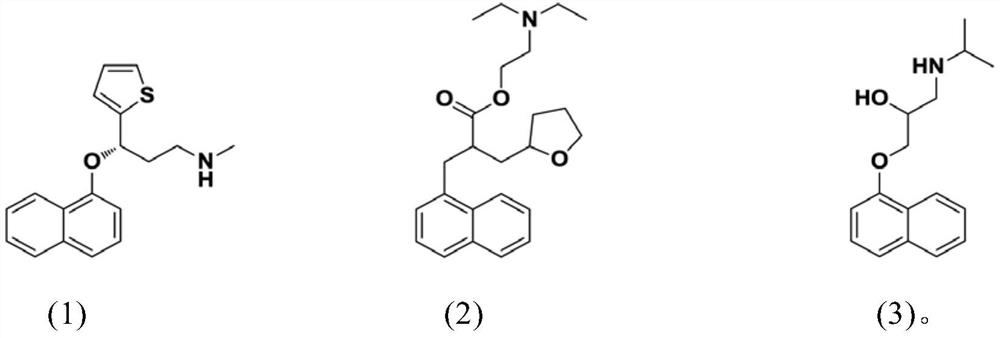
[0096] Using the method described above, compounds 1, 2 and 3 were subjected to IC 50 Inhibitory Activity Test.
[0097] The results are shown in Table 1 below: the compounds 1, 2 and 3 of the present invention are all transient receptor potential channel protein (TRPA1) activity inhibitors, and the compounds 1, 2 and 3 have significant inhibitory effect on the activity of TRPA1.
[0098] Table 1
[0099]
Embodiment 2
[0101] pill box
[0102] Prepare following a kind of medicine box, described medicine box comprises:
[0103] (1) a first container, and a first pharmaceutical preparation (tablet) located in said container, which preparation contains the following active ingredients;
[0104]
[0105] (2) a second container, and a first pharmaceutical preparation (such as a tablet) located in said container, which preparation contains the following active ingredients;
[0106]
[0107] and (3) instruction manual.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com