Methods for treating hcv
A patient and compound technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antiviral agents, etc., can solve problems such as curative effect and tolerance limitations, and inability to eliminate viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0090] Example 1. Treatment of HCV genotype 1b without interferon and without ribavirin
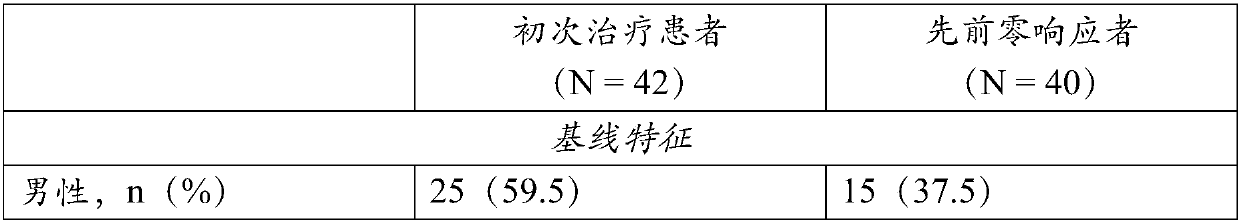
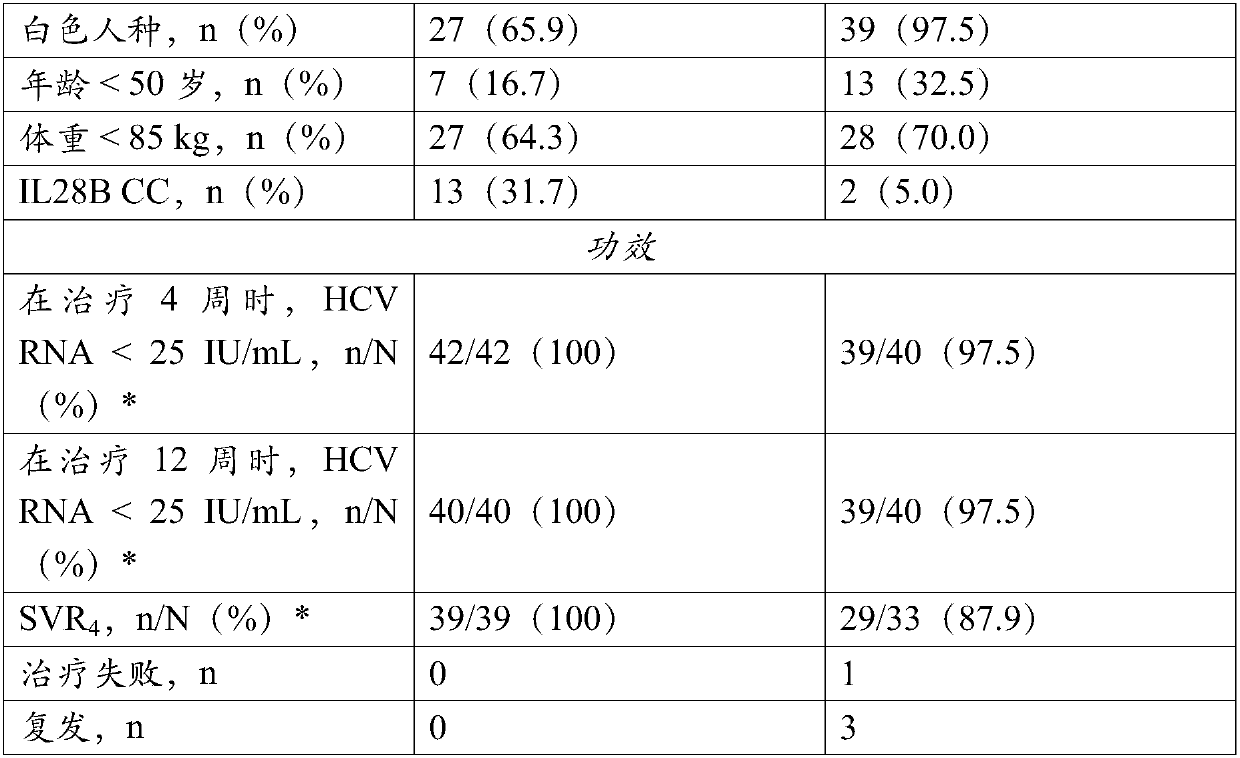
[0091] Treatment-naive patients and patients who were previously pegIFN / RBV null responders received compound 1 (150 mg QD), ritonavir (100 mg QD) and compound 2 (25 mg QD) for 12 weeks. Forty-two treatment-naive patients and 40 previous pegIFN / RBV null responders with chronic HCV genotype 1b infection were enrolled. All patients were non-cirrhotic. Baseline characteristics are shown in Table 1. Observed rates of HCV RNA 4 Rates (percentage of patients with HCV RNA 4 rate of 100%, and among previously zero responders, SVR 4 The rate is 87.9%.
[0092] Further follow-up showed that after 8 weeks of treatment, 100% of the 39 actually tested treatment-naive patients had no detectable HCV RNA; and after 12 weeks of treatment, among the 30 actually tested treatment-naive patients Among them, 97% of patients (29 / 30) had no detectable HCV RNA. Follow-up testing showed SVR was achieved in 40...
example 2
[0099] Example 2. Clinical modeling for interferon-free treatment of HCV genotype 4
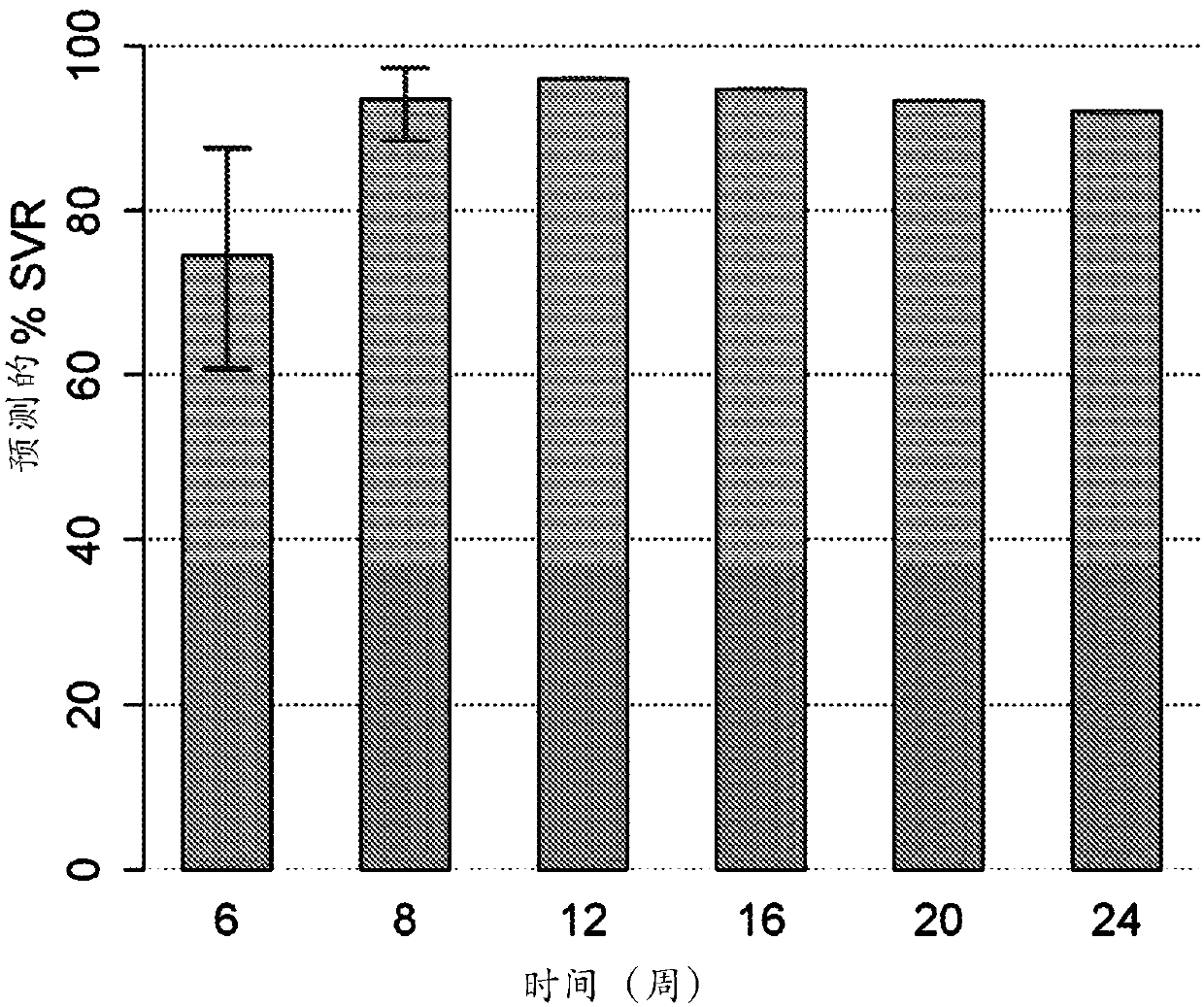
[0100] A novel clinical model for evaluating the appropriate dose and duration of interferon-free HCV therapy using a combination of various DAAs has been described in Example 6 of US Patent Application Publication No. 2013 / 0102525. The data from clinical studies of Compound 1 and Compound 2, together with in vitro confirmatory experiments, were used to estimate the pharmacokinetic model parameters and viral kinetic model parameters. Based on the relationship between the in vivo and in vitro data for genotype 1, the in vivo parameters for genotype 4 were roughly estimated using the in vitro data. The model predicted that more than 90% of genotype 4 naive patients could achieve SVR after 8 or 12 weeks of dosing with the combination of compound 1 (150 mg QD), ritonavir (100 mg QD) and compound 2 (25 mg QD). see figure 1 . figure 1 Shown are the predicted median SVR percentages ("%SVR") and 9...
example 3
[0101] Example 3. Clinical study of interferon-free treatment of HCV genotype 4
[0102] A clinical study of interferon-free treatment of HCV genotype 4 was performed. In this study, two groups of treatment-naive patients with HCV GT 4 infection were enrolled, each group comprising approximately 40 patients. Compound 1 (150 mg QD), ritonavir (100 mg QD) and Compound 2 (25 mg QD) were administered to each patient in both groups. Patients in the first group were also given weight-based ribavirin, but not the second group. The baseline characteristics of these patients are summarized in Table 2.
[0103] After 12 weeks of treatment, patients in the first group (with ribavirin) achieved an SVR12 rate of approximately 100%, and patients in the second group (without ribavirin) achieved an SVR12 of approximately 90%.
[0104] Table 2.
[0105]
[0106] In another group, 49 interferon partial / null responders or relapsers with HCV GT 4 infection were enrolled and treated with co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap