Use of spiroketal polyacetylene compounds in the preparation of anti-efflux pump drug-resistant Staphylococcus aureus sensitization drugs
A technology of polyacetylenes and spiroketals, applied in the field of pharmacy, can solve the problems of limiting the clinical application of oxacillin, serious bacterial resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
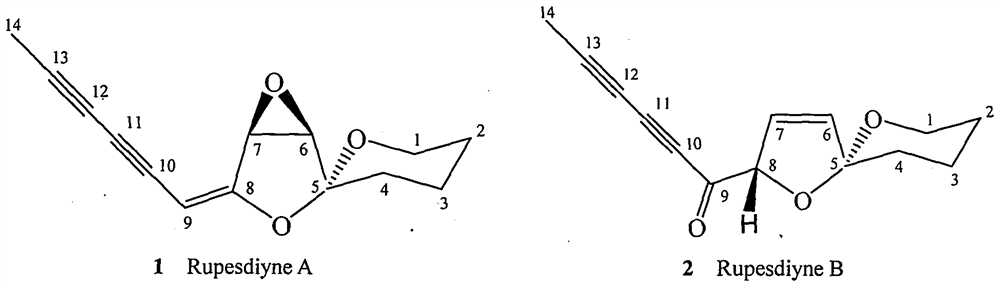
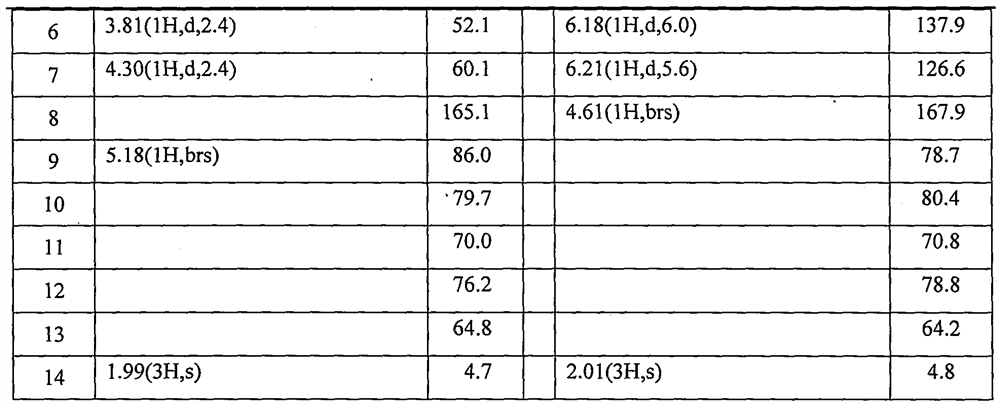
[0025] Example 1. Isolation of synergistic compounds Rupesdiyne A and Rupesdiyne B from plant Artemisia xinjiang
[0026] 27.3 g of petroleum ether extract from the 95% ethanol extract of Artemisia rupestris L., a plant of the genus Artemisia in the genus Compositae, was mixed with 200-300 mesh silica gel for column chromatography, and each 20 mL was a fraction. It was eluted with petroleum ether-dichloromethane (7:3), and fractions 8-54 were collected and marked as Fr01-12. Fr01-8 was separated and eluted with petroleum ether-dichloromethane (7:3), and fractions 10-18 and fractions 18-35 were collected and designated as Fr01-8-10 and Fr01-8-18, respectively. After being evaporated to dryness under reduced pressure, the weights were 3.2 g and 2.4 g, respectively. Fr01-12-2 and Fr01-12-23 were purified several times respectively to obtain a white solid Fr01-8-10-2 weighing 37.1mg, and a light yellow solid Fr01-8-18-6 weighing 68.9mg, which were obtained by thin-layer chromatog...
Embodiment 2
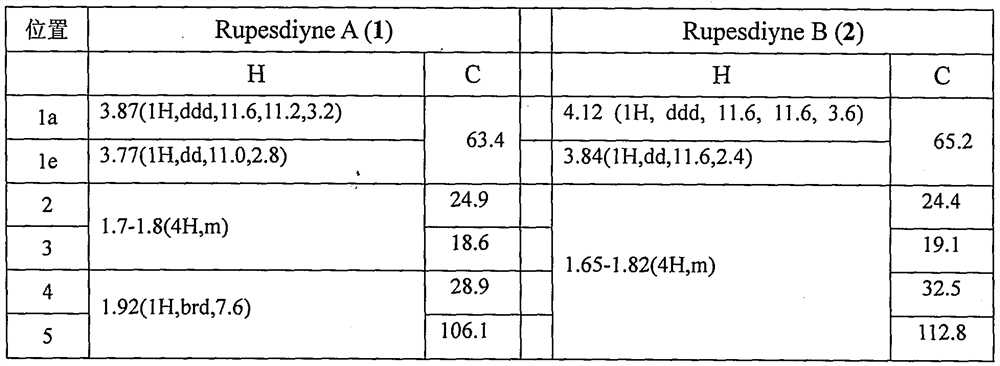
[0027] Example 2. Isolation of synergistic compounds Rupesdiyne A and Rupesdiyne B from the plant Chrysanthemum schizophrenia.
[0028]Ajania przewalskii Poljak (Ajania przewalskii Poljak), a plant of the genus Asteraceae, is dried and pulverized, and then repeatedly extracted with a ternary mixed solvent (petroleum ether: ether: methanol = 1:1:1) 36g of extract. Part of this extract (26 g) was mixed with 200-300 mesh silica gel for column chromatography, and was eluted with a gradient of petroleum ether-acetone system to obtain six components of Fr-1 to Fr-6. Fr-3 (1.9g) was further separated by silica gel column chromatography, and eluted with petroleum ether-dichloromethane (8:2) to obtain eight components of Fr-3-1~Fr-3-8. Continue to separate Fr-3-4 (820mg) by silica gel column chromatography, elute with petroleum ether-dichloromethane, separate and purify repeatedly, and finally further separate and purify by reverse phase silica gel column chromatography and normal phas...
Embodiment 3
[0033] Embodiment 3, the synergistic effect test of the combination of compound and oxacillin
[0034] The synergistic antibacterial test of compounds and antibiotics was carried out on a 96-well plate at different concentrations formed by the double dilution method;
[0035] An appropriate amount of oxacillin is dissolved in DMSO to prepare an antibiotic mother solution, and an appropriate amount of oxacillin mother solution is dissolved in broth to prepare an oxacillin stock solution. Samples of compounds Rupesdiyne A and Rupesdiyne B were dissolved in DMSO according to the calculated amount to prepare mother liquor. Diluted in broth, the initial inhibitory concentrations of the compound and antibiotic were 512 and 256 μg / mL, respectively. Carry out an orthogonal experiment with this, and record the concentration of oxacillin corresponding to the microwells where bacteria do not grow in each row is the MIC value of oxacillin when used in combination with the compound sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com