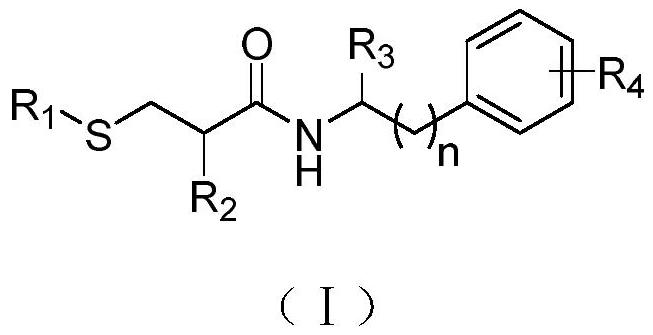
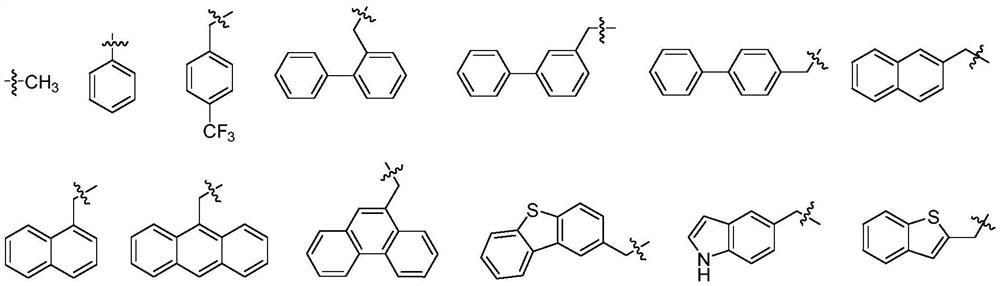
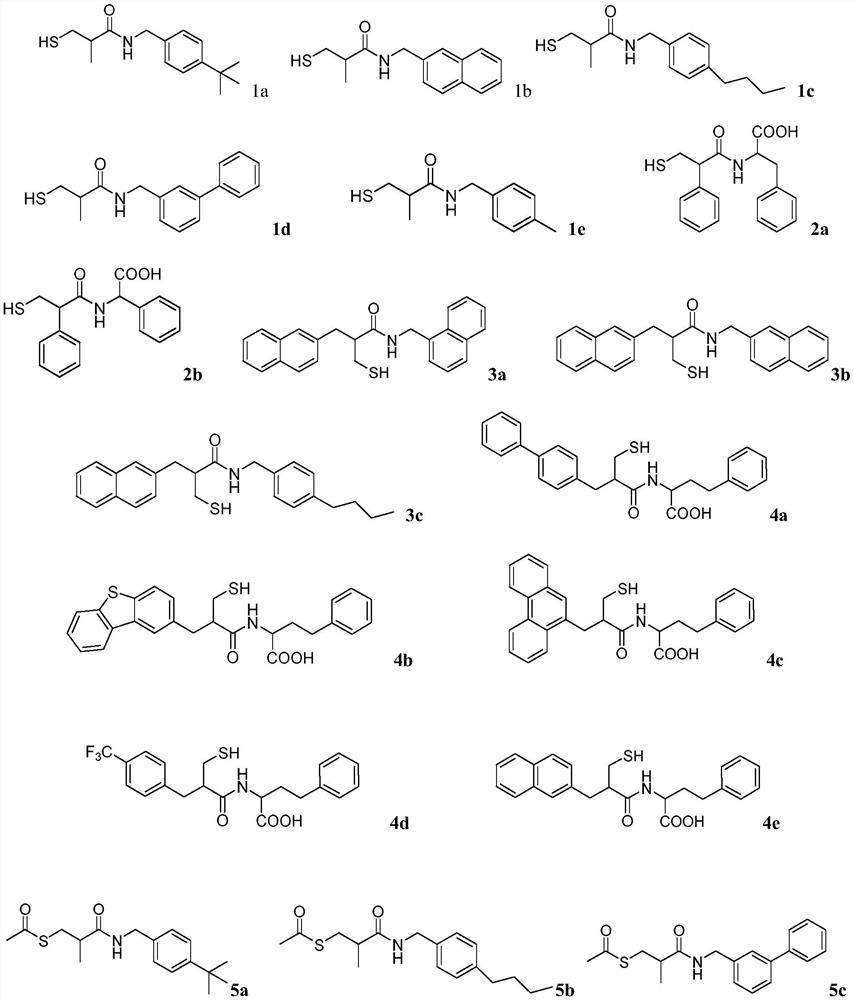
Mercaptopropionamide compounds, preparation method and medicinal use thereof
A technology of mercaptopropionamide and compound, applied in antibacterial drugs, organic chemistry, pharmaceutical formulations and other directions, can solve problems such as unreported anti-gram-negative bacteria activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]Example 1: 2 - (((1,1'-biphenyl) -4-yl) methyl) acrylate
[0055]
[0056]It is weighted 4-boron acid 500 mg (2.5 mmol), 2-bromoethacrylate 400 mg (2.1 mmol), 7.0 mg (0.02 mmol) of palladfluoroacetate and 177 mg (3.1 mmol) of potassium hydroxide (3.1 mmol) dissolved in 20 ml water After stirring at 90 ° C for 3 hours, it was extracted 3 times with dichloromethane, and the organic phase was mixed with anhydrous sodium sulfate, and the organic solvent was evaporated under reduced pressure, and the column chromatography was separated to obtain a colorless transparent oil liquid 527mg. 94.1% yield;
[0057]1H NMR (400MHz, CDCL3Δ: 7.60 (D, J = 7.2 Hz, 2H), 7.55 (D, J = 8.0 Hz, 2H), 7.41 (T, J = 7.4 Hz, 2H), 7.34 (S, 1H), 7.30 (D, J = 8.0Hz, 2H), 6.30 (S, 1H), 5.50 (S, 1H), 4.20 (DD, J = 7.2, 7.2 Hz, 2H), 3.71 (S, 2H), 1.26 (T, J = 7.2 Hz, 3H) .si-ms (m / z): 267.1 (m + h)+.
Embodiment 2
[0058]Example 2: 2 - ((1,1'-biphenyl) -4-yl) methyl) acrylic acid
[0059]
[0060]It is proud of the intermediate 2 - ((1,1,1, 1'-biphenyl) -4-yl) methacrylate 527 mg to 50 ml of tomato bottle, dissolved in 5 ml of tetrahydrofuran, and then add 3M aqueous sodium hydroxide aqueous solution 30ml, The reaction was stirred at 90 ° C for 4 h, and pH was adjusted to 2-3, and the mixture was extracted 3 times with 2M sulfuric acid, and the organic layer, sodium alhydrate sodium sulfate was combined, and the column chromatography was purified to give a white solid 429 mg, yield 90.9%;
[0061]1HNMR (400MHz, DMSO-D6Δ: 12.57 (S, 1H), 7.62 (DD, J = 7.6, 8.0 Hz, 4H), 7.45 (T, J = 7.4 Hz, 2H), 7.34 (T, J = 7.4 Hz, 1H), 7.29 ( D, J = 8.0Hz, 2H), 6.13 (S, 1H), 5.63 (S, 1H), 3.60 (S, 2H) .si-MS (M / Z): 239.1 (M + H)+.
Embodiment 3
[0062]Example 3: 3 - ((1,1'-biphenyl) -4-yl) -2 - ((acetyl) methyl) propionic acid
[0063]
[0064]3 ((1,1, 1'-biphenyl) -4-yl) methacrylic acid 429 mg, placed in a 50 ml of tomato bottle, add 20 ml of dichloromethane, ultrasound to dissolve the sample, then add catalytic amount Triethylamine, then dripped 1.8 ml (411 mg) of 10 ml of dichloromethane, which was diluted with 10 ml of dichloromethane, continued at room temperature and stirring at room temperature, and evaporated. Purification of acetic acid, column chromatography, gave 392 mg of white solid, yield 69.3%;
[0065]1H NMR (400MHz, CDCL3Δ: 11.15 (S, 1H), 7.48-7.35 (m, 6H), 7.26-7.21 (m, 3H), 3.09 (m, 1H), 3.00 (D, J = 7.2 Hz, 2H), 2.94 (D , J = 7.6Hz, 2H), 2.26 (s, 3h) .si-ms (m / z): 315.1 (M + H)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com