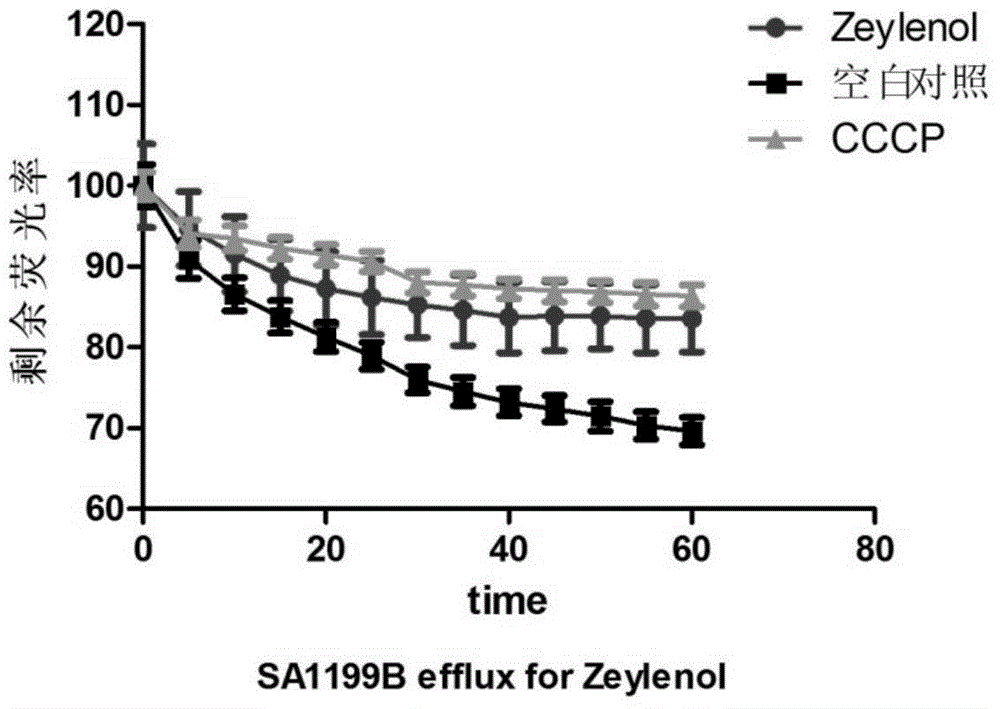
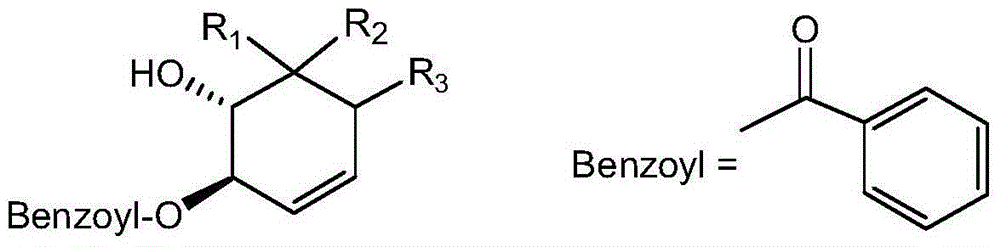
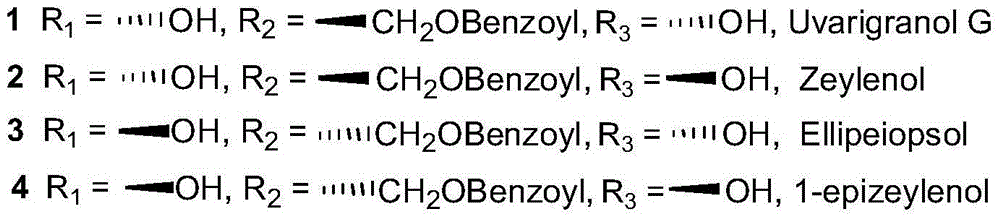Uses of benzoyl polyhydroxy cyclohexene in preparation of drug compositions
A technology of benzoyl polyhydroxycyclohexene and composition, which is applied in the field of preparation of synergistic pharmaceutical composition against ectopic drug-resistant Staphylococcus aureus, which can solve the problem of severe drug resistance of bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Isolation of synergistic compounds EllipeiopsolB and Zeylenol from the leaves of Piper betel
[0036] Take 3.5g extract from the chloroform extract of the 95% ethanol extract of Piperbetle, mix it with 200-300 mesh silica gel for column chromatography, and use 20mL as a fraction. It was eluted with chloroform-acetone (9:1), and fractions 32-36 were collected and marked as Fr02-32. Fr02-32 was separated and eluted with chloroform-methanol (95:5). Fraction 4-5 was collected and recorded as Fr02-32-4, and weighed 430 mg after evaporating to dryness under reduced pressure. Continue to separate Fr02-32-4, elute with chloroform-acetone (9:1), collect fractions 2-5 and fractions 6-10 respectively, and record them as Fr02-32-4-2 and Fr02-32-4 -6, evaporated to dryness under reduced pressure and weighed 160mg and 220mg respectively. Fr02-32-4-2 and Fr02-32-4-6 were purified several times respectively to obtain a white solid Fr02-32-4-2 weighing 20 mg and Fr02-32-4-6...
Embodiment 2
[0037] Embodiment 2, isolating synergistic compound EllipeiopsolB and Zeylenol from plant floret purple jade disc
[0038] 3.34Kg of the dried aerial part of the plant of the genus Uvaria rufa was extracted with chloroform to obtain 482g of green extract. Part of this extract (150 g) was extracted and layered with 50% petroleum ether-alcohol / water solution, and then the alcohol / water layer was extracted with chloroform to obtain 104 g of chloroform sub-extract. 104g of the extract was separated by column chromatography and eluted with chloroform-acetone to obtain five components of Fr-1 to Fr-5. Fr-3 (5 g) was separated by silica gel column chromatography and eluted with chloroform-methanol to obtain nine components of Fr-3-1~Fr-3-9. Fr-3-5 (2.89 g) was further separated by silica gel column chromatography and eluted with dichloromethane-acetone to obtain thirteen components from Fr-3-5-1 to Fr-3-5-13. Fr-3-5-7 (1.22g) was further separated and purified by reverse phase sili...
Embodiment 3
[0043] Embodiment 3, synergistic compound Zeylenol is synthesized with simple chemical raw material
[0044] synthetic route:
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com