A kind of bean epoxyhydrolase mutant with improved stereoselectivity and its application
A technology for hydrolyzing enzymes and epoxides, which is applied in the field of enzyme engineering, can solve the problem that stereoselectivity cannot satisfy the production of high-enantiopure epoxides and vicinal diols, and achieve the effect of improving enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Mutant plasmid construction
[0030] The E.coli BL21(DE3) / pET-28a-pveh1 (Ye Huihua, Hu Die, Li Chuang, et al. New type of common bean Heterologous expression and enantio-normalized catalytic properties of epoxide hydrolase[J]. China Biotechnology, 2016, 36(10): 21-27. 169: 41-54.) Plasmids were extracted as unidirectional site-directed mutagenesis template,
[0031]The primers used were W102L-F (5'-3'GTTGCCCATGATCTCGGAGCCCTAGTA); W102L-R (TACTAGGGCTCCGAGATCATGGGCAAC).
[0032] use HS PCR enzyme (purchased from TaKaRa Company) adopts the method of whole plasmid PCR. Using the PvEH1 plasmid as a template, PCR conditions: denaturation at 98°C for 10 s; annealing at 55°C for 5 s; extension at 72°C for 3.5 min, 30 cycles, and extension at 72°C for 10 min. The PCR product was transformed into E.coli BL21 (DE3) competent cells by digesting the template plasmid with Dpn I enzyme, and the transformation was performed according to the instruction manual of the SK9...
Embodiment 2
[0033] Embodiment 2: the acquisition of mutant enzyme
[0034] The obtained mutant enzyme expression vector was inoculated (the inoculum size was 1%) in 2 mL of LB medium containing 1% kanamycin, at 37 ° C, 220 r min -1 Cultivate overnight; take 2mL culture medium and transfer to 100mL LB medium containing 1% kanamycin, cultivate to OD 600 When it is 0.6~0.8, add 100μL of IPTG (500mmol·L -1 ) to a final concentration of 0.5mmol L -1 After induction at 20°C for 10 h, the recombinant bacterial cells were collected by centrifugation. It was determined that the enzyme activity of the epoxide hydrolase of the bacteria to rac-pCSO was 27U / g of the bacteria.
Embodiment 3
[0035] Example 3: Analysis of initial substrate concentration for whole cell catalysis
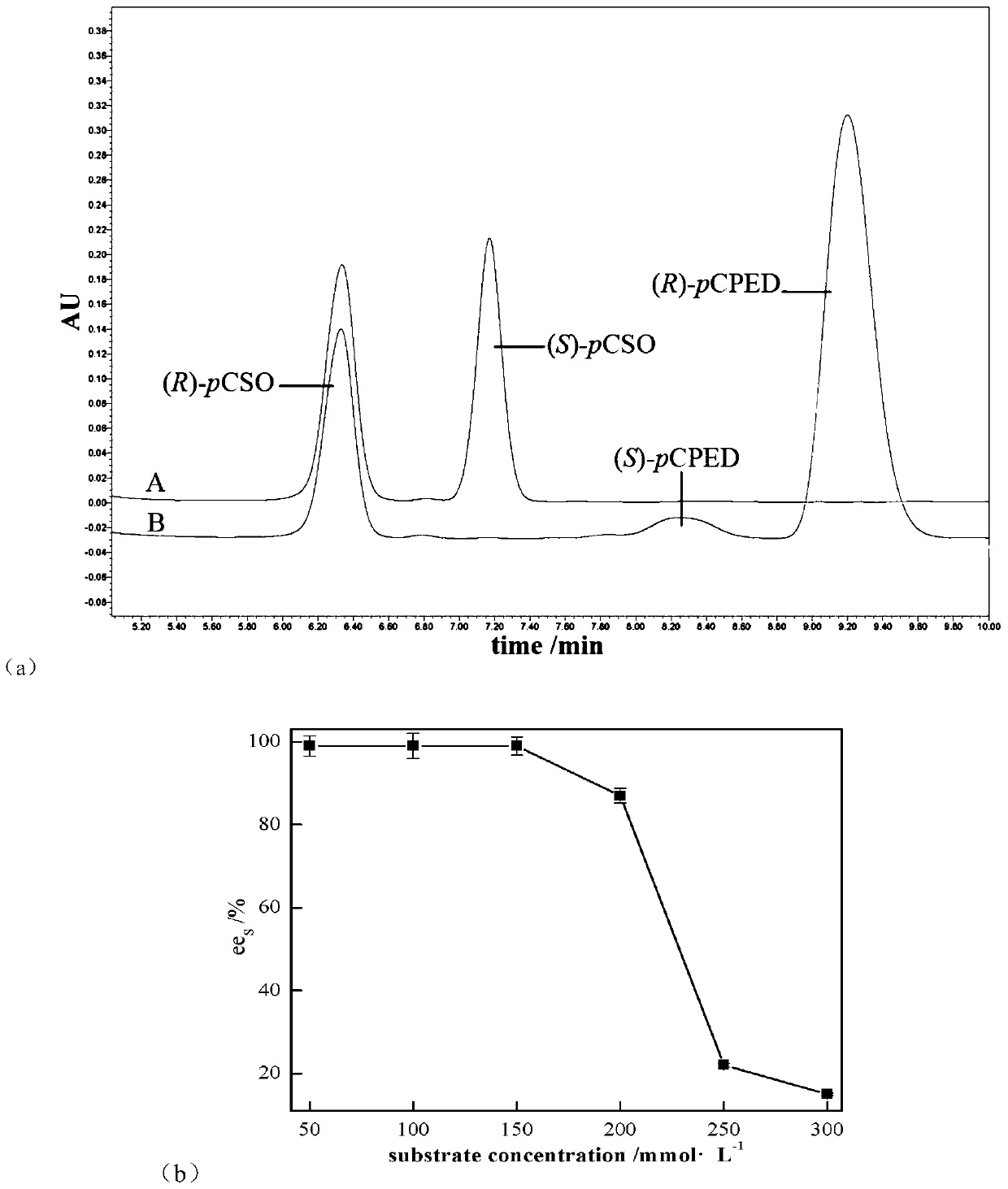
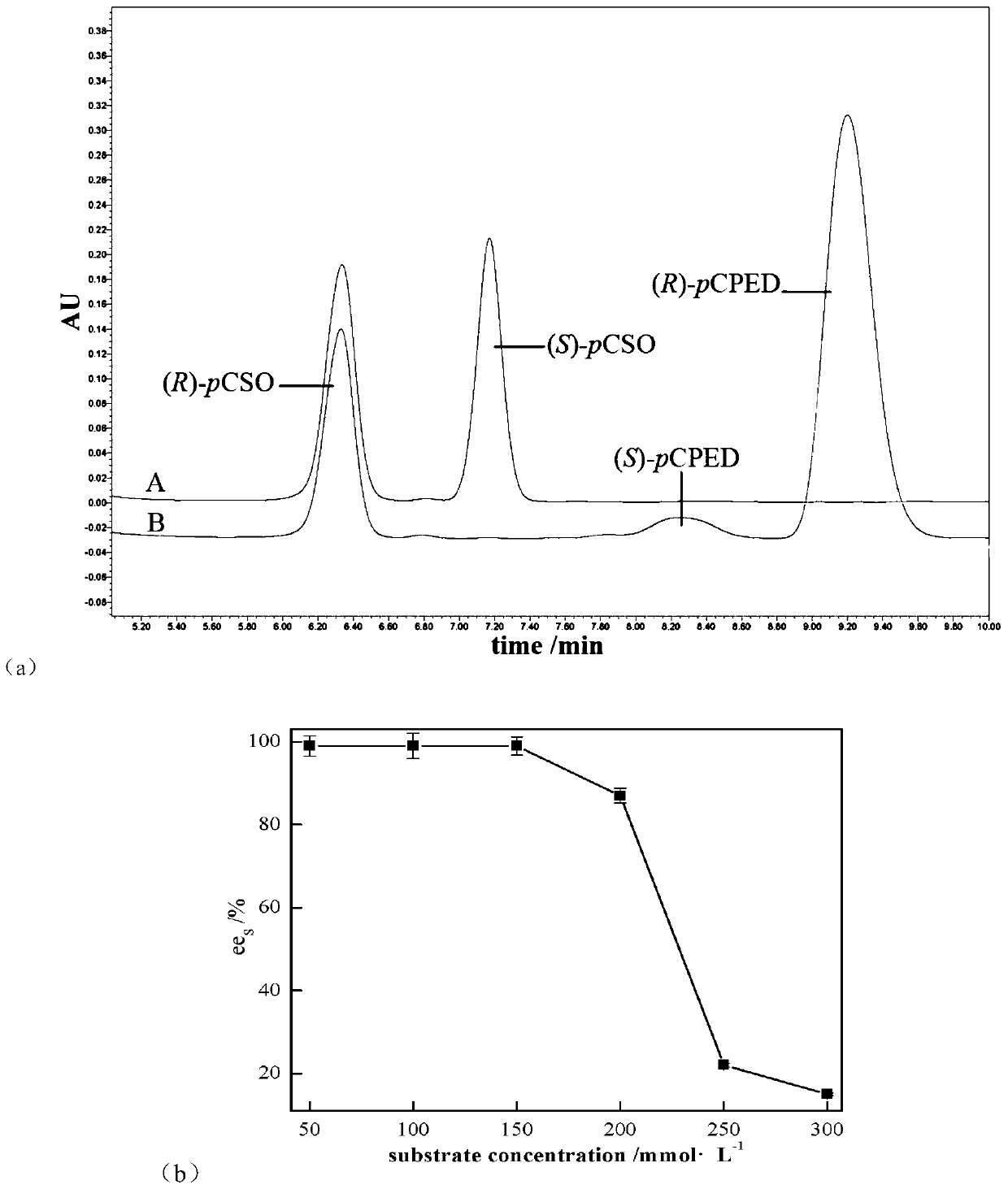
[0036] Add the wet bacterial suspension to the 2mL EP tube with a concentration of 200mg·mL -1 PvEH1 W102L Whole cells, substrate initial concentration from 0 to 300mmol·L -1 rac-pCSO, the buffer is sodium phosphate buffer (pH=7.0, 100mmol·L -1 ), the reaction system is 1mL, 25℃, 220r·min -1 After reacting in a constant temperature shaker for 12 hours, take 50 μL of the sample and extract it with 1 mL of ethyl acetate, dry the organic phase over anhydrous magnesium sulfate, and measure ee by HPLC s .
[0037] Such as figure 1 As shown in (a), using PvEH1 W102L No obvious by-products were observed during whole-cell catalyzed hydrolysis of rac-pCSO, in which (S)-pCSO was catalyzed by PvEH1 W102L Preferentially catalyzes hydrolysis to (R)-pCPED.
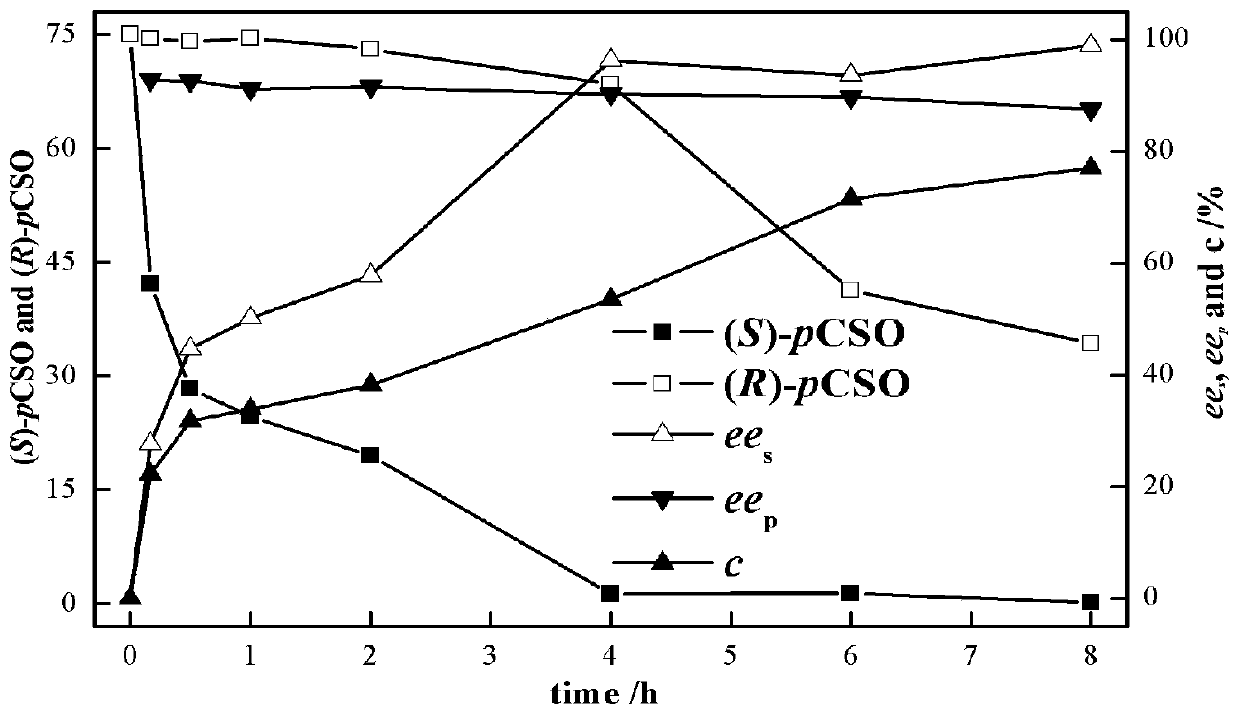
[0038] Use a final concentration of 200mg·mL -1 PvEH1 W102L Separately split the initial concentration from 50 to 300mmol·L -1 Different...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com