HIV vaccines comprising one or more population episensus antigens
A technology of HIV-1, epitope, applied in the field of HIV vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
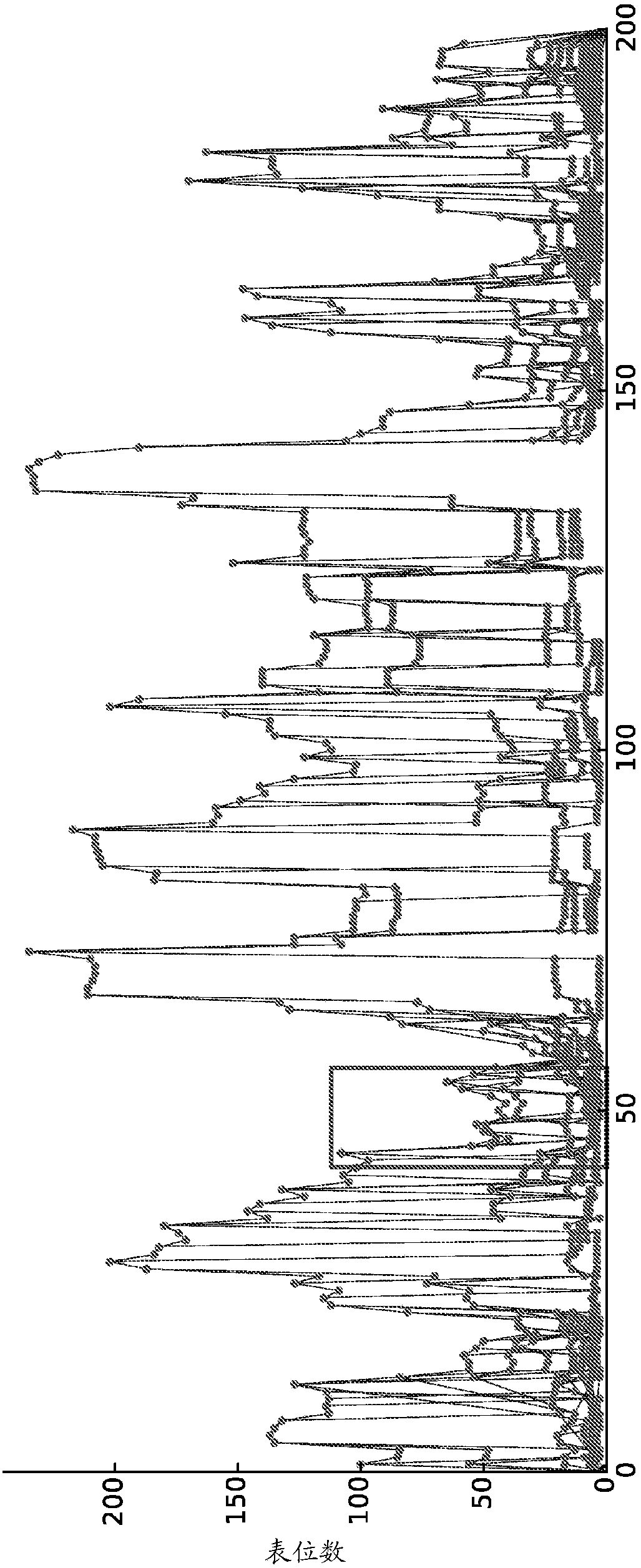
[0084] Example 1: Graphical Model of Optimal Epitope Coverage of Aligned Sequences.
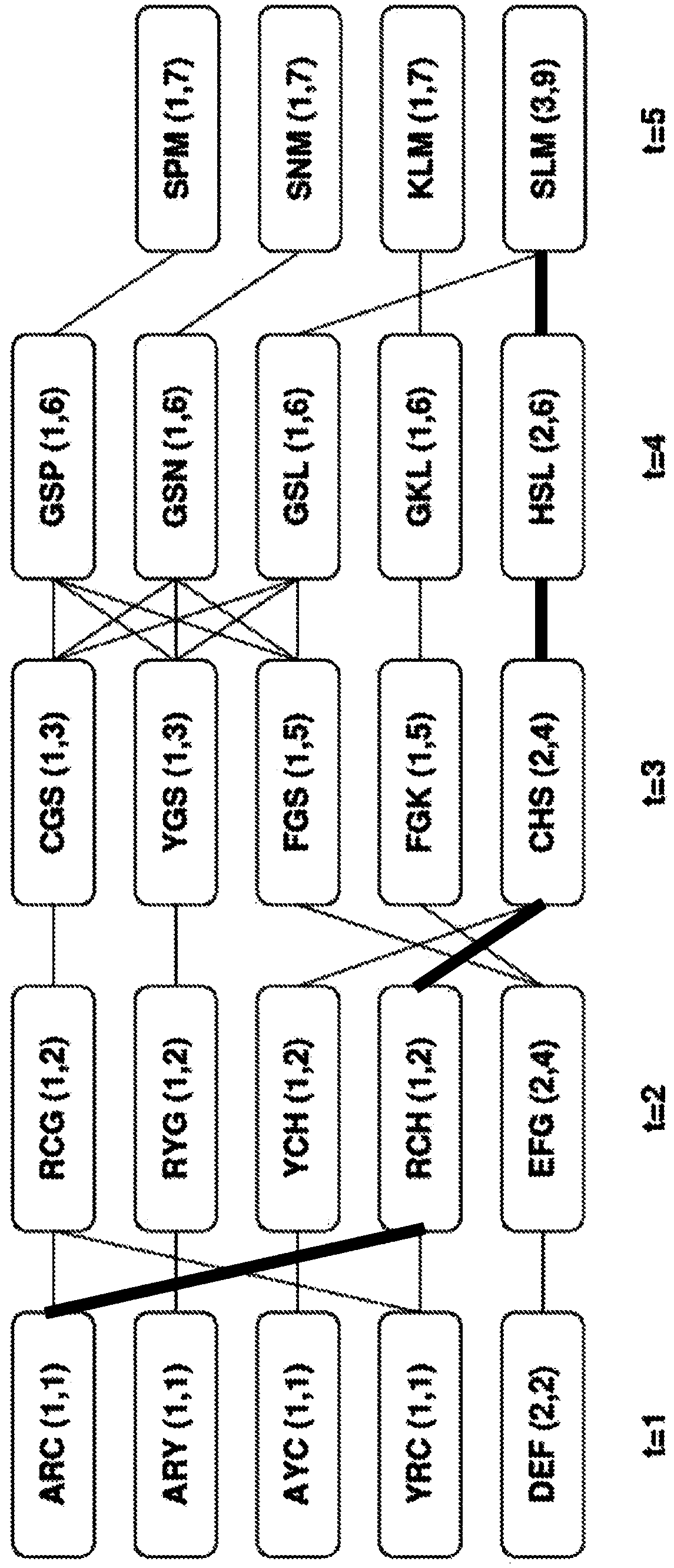
[0085] Suppose there is a set S={s of N aligned sequences 1 ,s 2 ,...,s N}. Each sequence is a string of letters (corresponding to the twenty amino acids, and possibly additionally a few special characters corresponding to gaps, unknowns, etc.). Due to sequence alignment, all sequences have the same length T, position t=1...T is well defined from sequence to sequence; s n [t] will be written as the tth character in the nth sequence. For a subsequence of s starting at position t and ending at position u, it is useful to introduce the notation s[t:u].
[0086] Potential epitopes are defined as short sequences of k characters, usually 8 to 12 characters. The epitopes studied are subsequences of the sequences in S. In fact, the sequence s can be thought of as a list of epitopes: e 1 ,...,e T-k+1 , where e t =s[t:t+k-1]. Note, however, that for a list of sequence-related epitopes, the e...
example 2
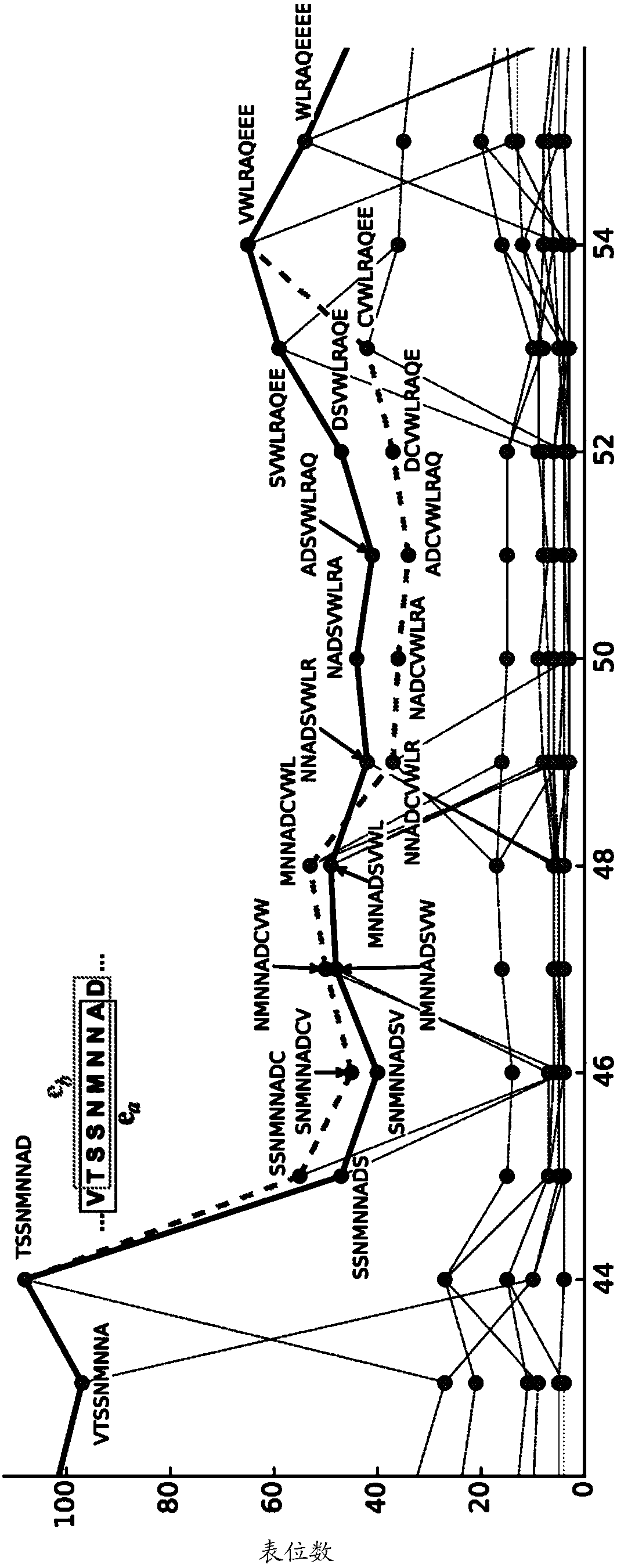
[0136] Example 2: Graphical Model for Optimal Epitope Coverage of Unaligned Sequences
[0137] Take a set of N unaligned protein sequences S={s 1 ,s 2 ,...,s N} to characterize virus variability within a target population (e.g., phylogenetic clade, national or global). A potential epitope is a subsequence of k amino acids (usually k=9). Each potential epitope e is assigned an integer frequency f(e) corresponding to the number of sequences in S in which e occurs. The monovalent problem is to design a single artificial sequence q that resembles the natural protein but optimally covers the potential epitopes in population S. Writing E(q) as the set of epitopes occurring in q, our measure of coverage is
[0138] Coverage (q) = ∑ e∈E(q) f(e) / ∑ e∈E(s) f(e)
[0139]The numerator is the sum of the frequencies of epitopes appearing in q, and the denominator is normalized by the sum of all epitopes appearing in any sequence in S. This equation can be represented as a directed g...
Embodiment 3
[0142] Example 3: Custom Therapeutic Vaccines.
[0143] While it is not possible to build a designer vaccine for each subject, the viruses from that subject can be sequenced to try to get a good match from a small benchmark set of vaccine options. The first thing to consider is the experimental population based on the US clade B, which is focused on Gag proteins. A South African-based baseline vaccine set and a global vaccine set were designed, along with an updated US-based B clade design. p24 is the most highly conserved subprotein of Gag and can be excised from the larger Gag protein to provide a conserved region approximately 230 amino acids in length. A conserved region approach has also been considered as an alternative to Gag, perhaps focusing on regions in Gag and Pol, which may include conserved segments of Nef as well as any other protein of interest.
[0144] This is a very different optimization problem than trying to design an ensemble that provides optimal popu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com