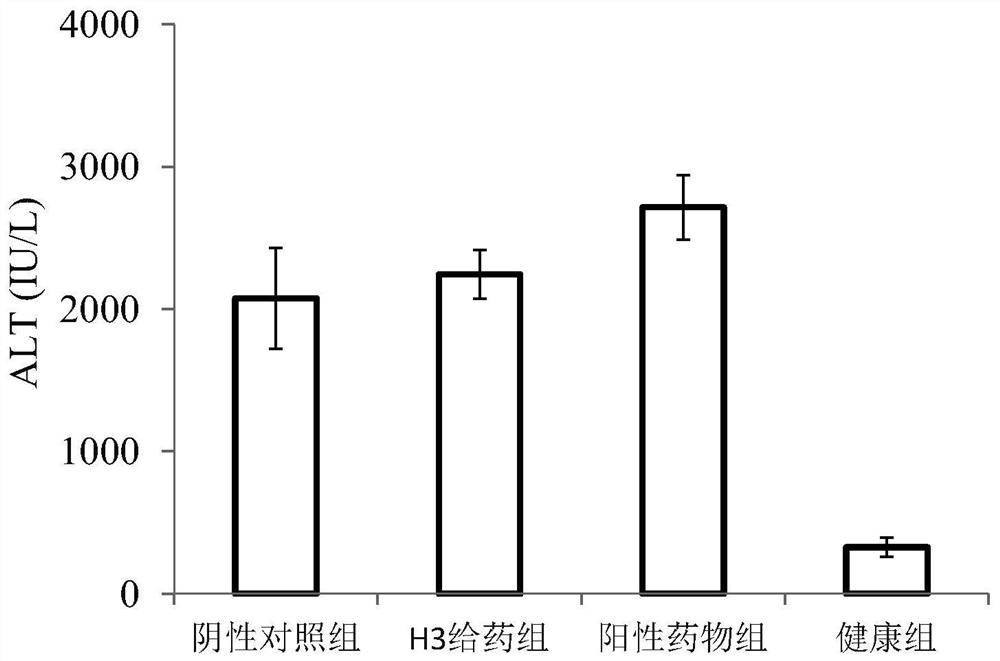
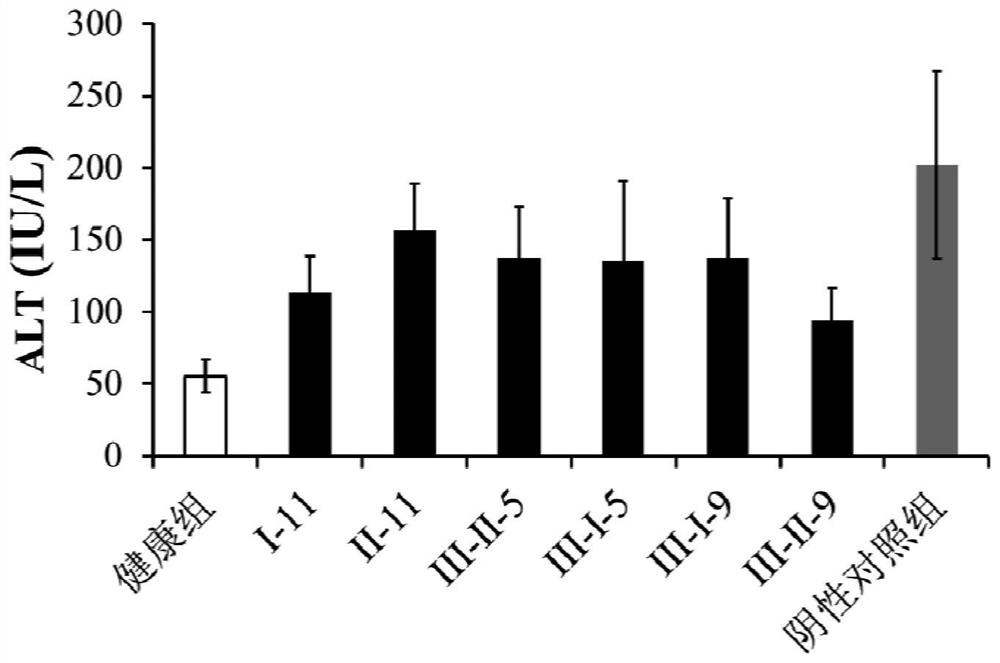
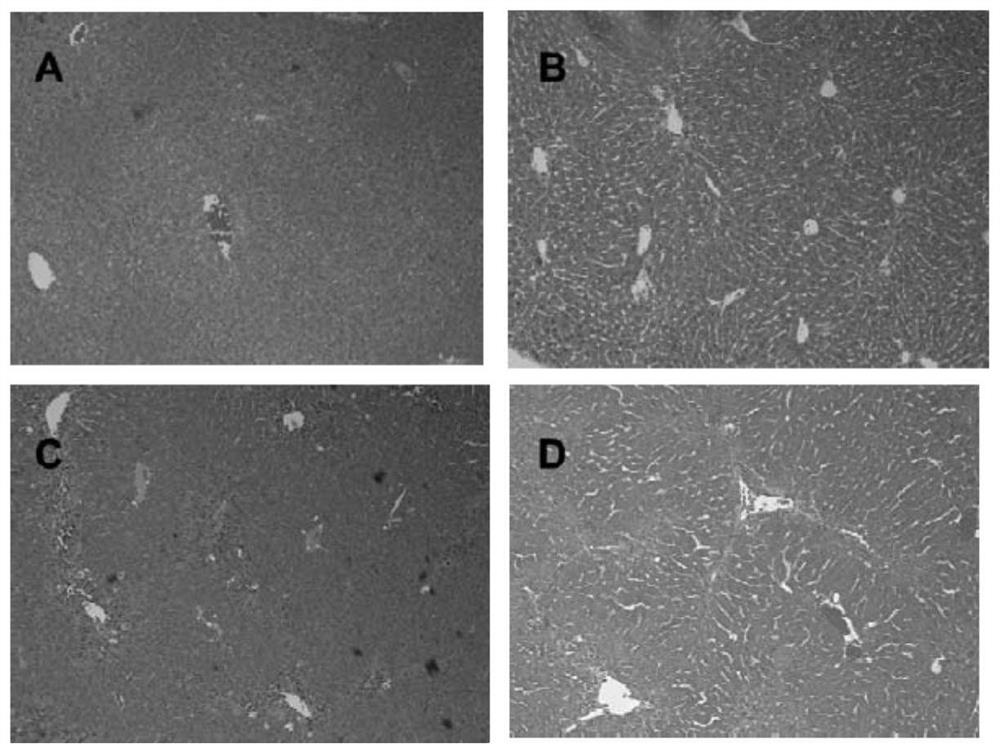
low molecular weight heparin
A low molecular weight heparin, molecular weight technology, applied in the field of low molecular weight heparin, can solve problems such as lack of treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of low molecular weight heparin 1 (hereinafter, also referred to as low molecular weight heparin I-11)
[0071] Combine 5g heparin (purchased from Changshan Biochemical Pharmaceutical Co., Ltd., product name: heparin sodium, with a molecular weight distribution of 5,000 to 30,000, with an average molecular weight of 20,000) and 100 mL Tris buffer (20mM Tris, 50mM NaCl, 20mM CaCl) 2 ) Mix and prepare a 50 g / L heparin solution (pH 7.6), and add heparinase I prepared according to ZL200410038098.6 with a total enzyme activity of 100 IU to 100 mL of the prepared 50 g / L heparin solution. Use a quartz cuvette with an optical path difference of 1 cm and an ultraviolet spectrophotometer to monitor the solution's light absorption A231 at 231 nm. When A231 reaches the reaction control point (A231=46.3), the reaction is completed. The method of ending is to inactivate the enzyme in the reaction solution in a boiling water bath at 100°C for 5-10 minutes, and then t...
Embodiment 2
[0076] Example 2 Preparation of low molecular weight heparin 2 (hereinafter, also referred to as low molecular weight heparin III-II-5)
[0077] To 100mL of 50g / L heparin solution prepared in the same way as in Example 1, an excess of heparinase III (215IU) prepared in accordance with ZL201010259913.7 was added to the end of the reaction, and ultraviolet absorption spectroscopy (A231) was used to determine the heparinase reaction. End point (control at A231=20), and then add heparinase II (20IU) prepared according to the ZL201010259905.2 method, and when A231 reaches the reaction control point (A231=61), the reaction is ended. The method to end is to take a certain volume of the reaction solution (300ml), inactivate it in a boiling water bath at 100°C for 5-10 minutes, cool to room temperature, add 6 times the volume of absolute ethanol, stir at room temperature for 10 minutes, stir for 10 minutes, and then at room temperature Centrifuge at 4000r / min for 15min, collect the precip...
Embodiment 3
[0079] Example 3 Preparation of low molecular weight heparin 3 (hereinafter, also referred to as low molecular weight heparin II-11)
[0080] To 100 mL of a 50g / L heparin solution prepared in the same way as in Example 1, heparinase II prepared according to ZL201010259905.2 with a total enzyme activity of 100 IU was added at one time. Use a quartz cuvette with an optical path difference of 1 cm and an ultraviolet spectrophotometer to monitor the solution's light absorption A231 at 231 nm. When A231 reaches the reaction control point (A231=46.5), the reaction is completed. The method of ending is to inactivate the enzyme in the reaction solution in a boiling water bath at 100°C for 5-10 minutes, and then take out the reaction system and cool it to room temperature. Add 6 times the volume of absolute ethanol, stir at room temperature for 10 minutes, then centrifuge at 4000r / min for 15 minutes at room temperature, collect the precipitate, add deionized water 2 to 3 times the mass of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap