A Method for Predicting the Reduction Rate Constant of Nitroaromatic Compounds
A technology of nitroaromatics and rate constants, which is applied in the field of ecological risk assessment testing strategies, can solve the problems of poor model stability and inaccurate prediction of nitroaromatics kre, etc., and achieve the effect of low cost and labor saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
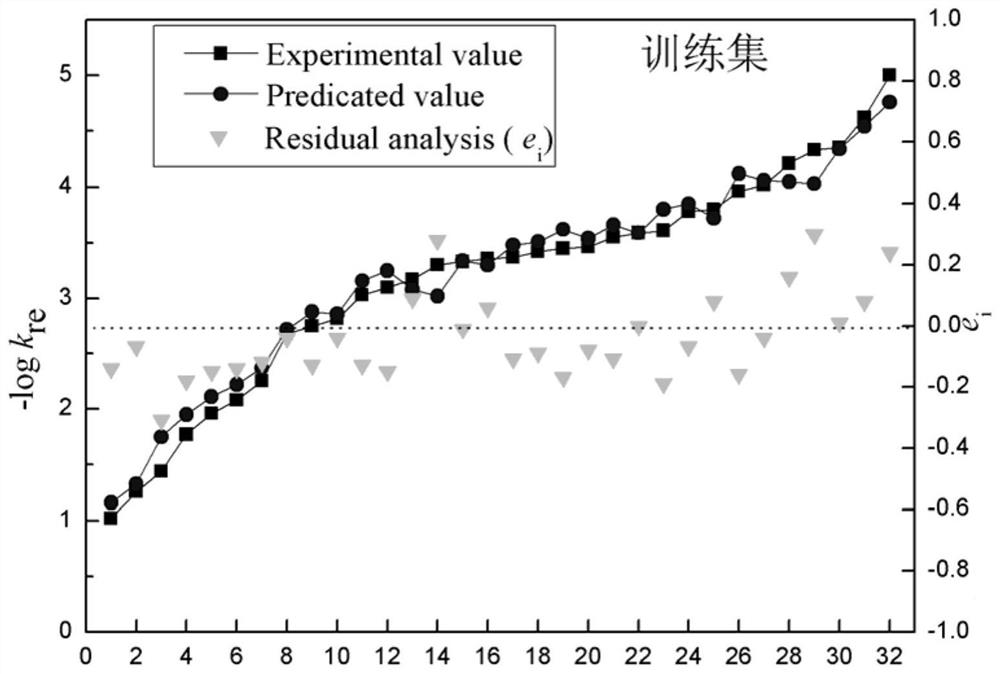
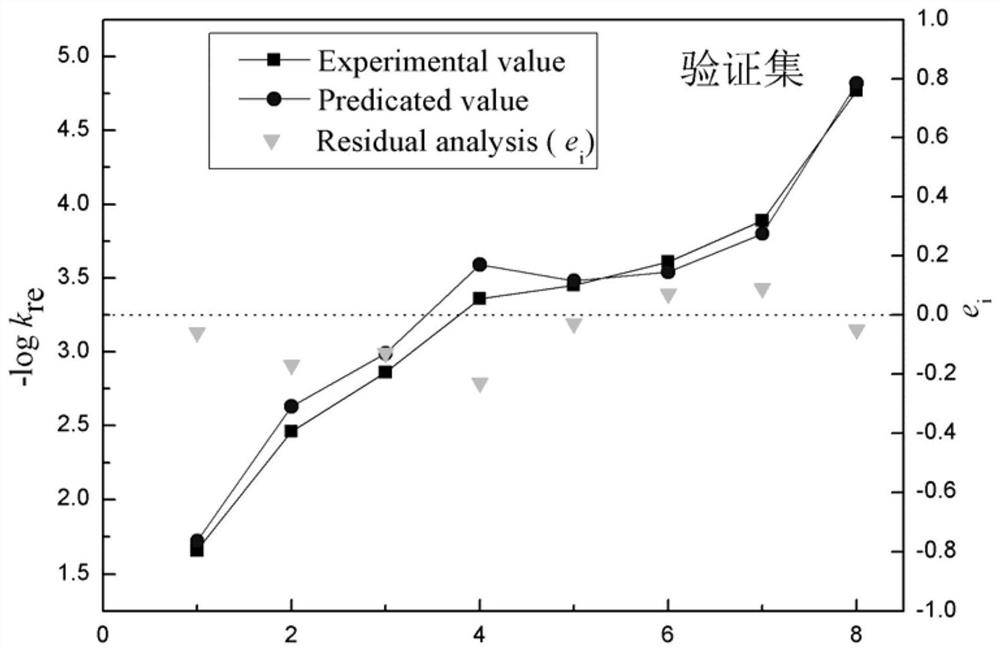
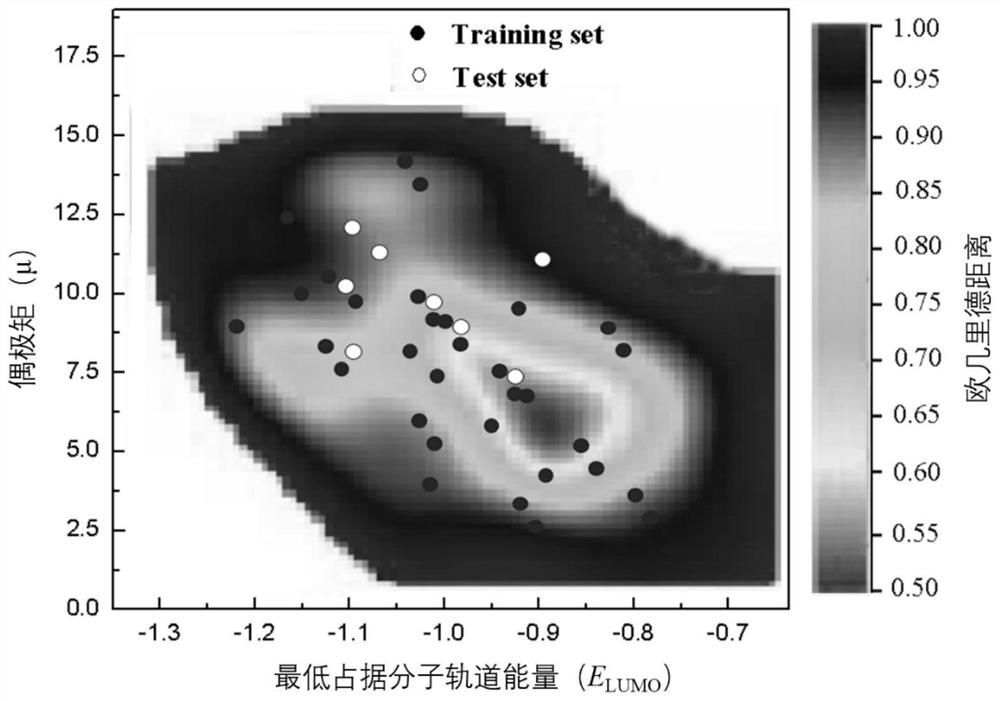
Image
Examples
Embodiment 1
[0040] Embodiment 1: Predict the -log k of o-nitrobenzoic acid catalytic reduction with the prediction model of the present invention re value
[0041] Taking the activated carbon catalytic reduction of o-nitrobenzoic acid as an example, the structural information of the p-nitrobenzoic acid molecule was first checked, and then the geometric structure was optimized by using the quantum chemistry software Gaussian 09 package to obtain the monocyclic nitroaromatic structure The optimal configuration of , so as to obtain the molecular descriptor I required for its model P , Q c ,E HOMO ,E LUMO The values of , ΔE and μ are 10.26, -0.301, -10.259, -1.139, 9.120 and 6.334, respectively. Then it was characterized by the Euclidean distance method in AmbitDiscovery (application and characterization software), and it was found that the compound was within the application domain of the model, so the model could be used to predict the reduction rate constant of nitrobenzoic acid. Su...
Embodiment 2
[0044] Embodiment 2: Application of the constructed prediction model to predict -log k of 2,4-dinitrochlorobenzene reduction re value
[0045] According to the structural information of 2,4-dinitrochlorobenzene, after using the Gaussian 09 program package to optimize the structure of the compound, its molecular descriptor I can be obtained P , Q c ,E HOMO ,E LUMO The values of , ΔE and μ are 17.80, -0.308, -10.898, -1.809, 9.089 and 1.466, respectively. Then according to the Euclidean distance method, it is found that it is within the scope of the model application domain, so this model can be used to predict the -log k of 2,4-dinitrochlorobenzene re value. Substituting the values described above into the established QASR model linear relational formula, the -log k of 2,4-dinitrochlorobenzene reduction is obtained re The predicted value is 2.22, the -log k found in the literature "Russ.J.Electrochem.2010,46(8):934-940" re The experimental value is 2.08, and the erro...
Embodiment 3
[0046] Embodiment 3: Application of the constructed prediction model predicts -log k of picric acid (TNP) reduction re value
[0047] According to the structural information of TNP found, use the Gaussian 09 program package to optimize the structure of the compound, and then obtain its molecular descriptor I P , Q c ,E HOMO ,E LUMO The values of , ΔE and μ are 27.02, -0.382, -10.595, -1.34, 9.255 and 1.69, respectively. Then according to the Euclidean distance method, the compound is within the application domain of the model, so the model can be used to predict the -log k of TNP reduction re value. Substitute the quantum chemical parameters obtained above into the regression equation in this model to obtain the -log k of TNP reduction re The predicted value is 3.08, and the experimental value corresponding to TNP reduction found in the literature "Appl. Surf. Sci. 2015,331:210–218" is 3.17, with an error of 0.09, and the predicted value is very close to the experiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com