Adipose tissue derived mesenchymal stromal cell conditioned media and methods of making and using the same
An adipocyte and matrix technology, applied in cell culture medium, biochemical equipment and methods, tissue culture, etc., can solve problems such as difficulty in evaluating efficacy, dosage, stem cell host immune system attack, etc., and achieve the effect of storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
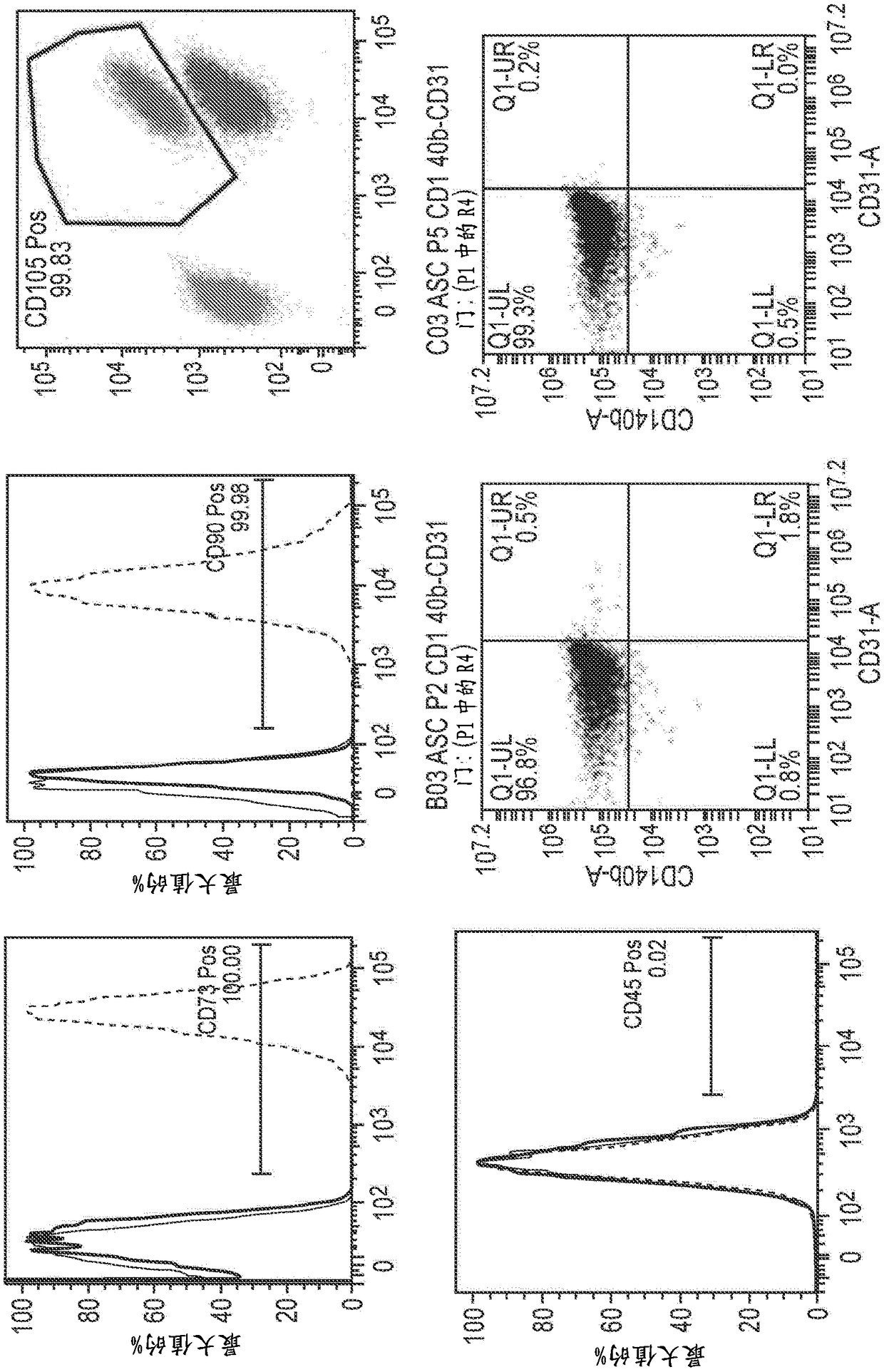
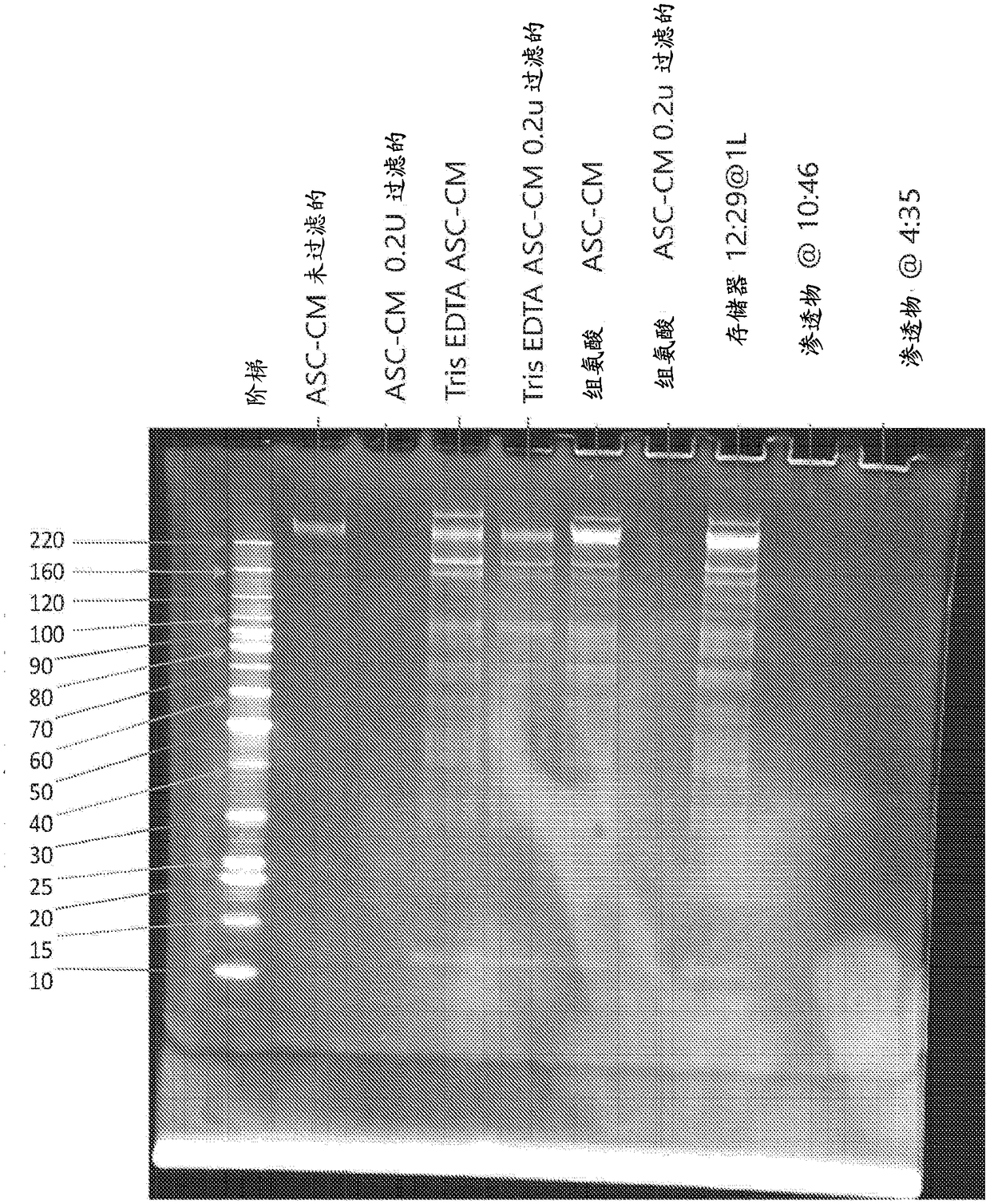
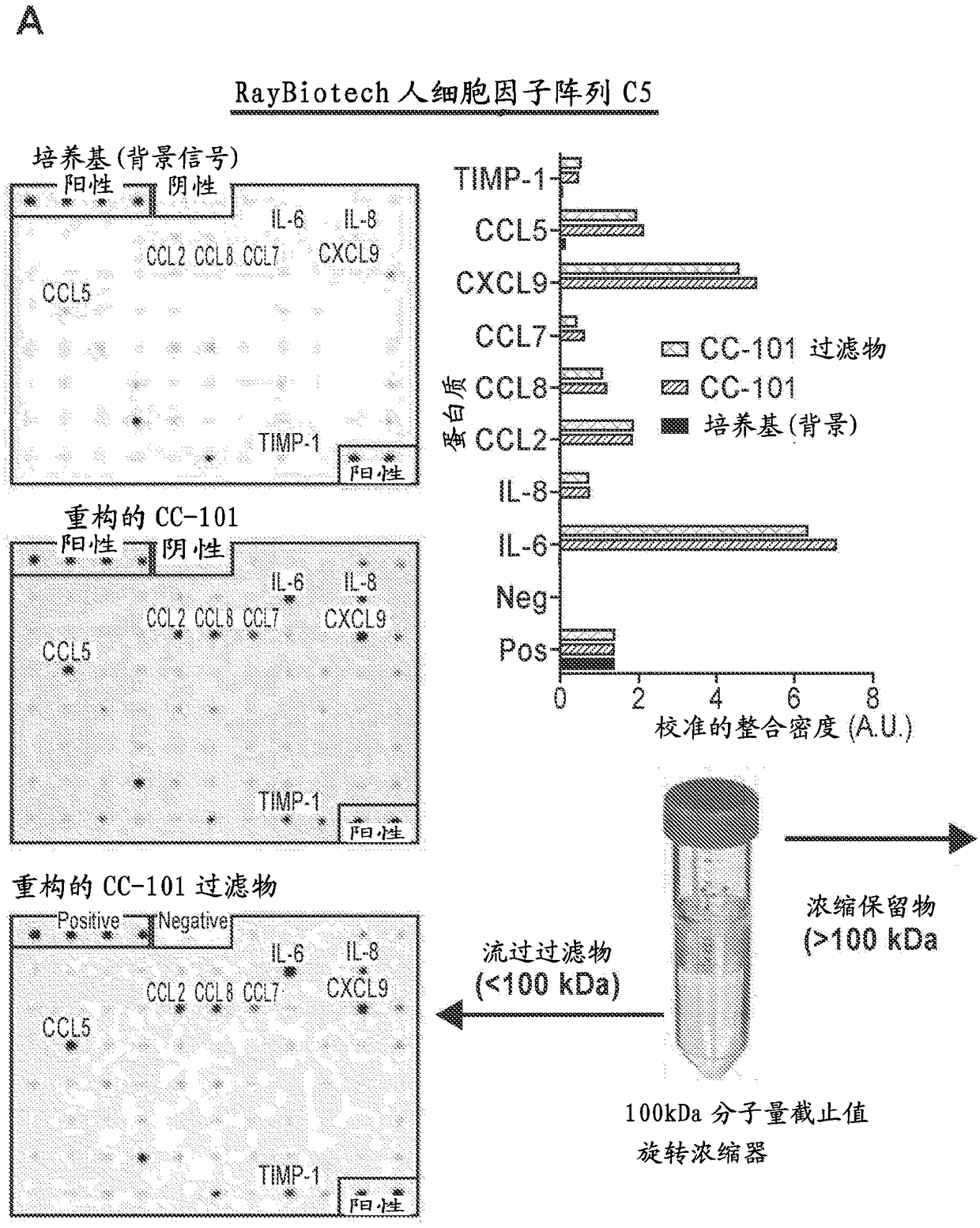
[0216]Example 1. Development of adipose tissue-derived mesenchymal stromal cell conditioned medium product under cGMP guidelines
[0217] Manufacturing plan
[0218] Donor selection and tissue harvest
[0219] About 150-300ml of abdominal tissue extracted by liposuction can be selected from suitable donors (such as non-smoking women under the age of 30, with a family history of longevity on both sides of the family, and / or without known diseases or chronic diseases Significant family history.
[0220] Digestion
[0221] Adipose tissue digestion was accomplished using minor modifications to standard tissue digestion protocols known in the art. Preferably, these changes are changes that help increase the overall POASC yield.
[0222] Approximately 300 ml of lipoaspirate was transferred to a sterile bottle with the adipose tissue above the blood fraction. Remove blood from beneath the adipose tissue using a 10 ml aspirator pipette and rinse the lipoaspirate with 300 ml DPB...
Embodiment 2
[0333] Embodiment 2: the preparation and testing of the pharmaceutical composition comprising lyophilized composition and slow-release drug delivery matrix
[0334] A 2 ml solution of the lyophilized composition prepared as outlined in Example 1 at a protein concentration of approximately 400 micrograms / ml was prepared and used as the starting solution for incorporation into the sustained release delivery matrix.
[0335] Macro matrices were formulated and release profiles were measured from six different matrices. An overview of the matrix is shown in the table below:
[0336]
[0337] Figure 10 Preliminary release data (expressed as percent payload) showing burst and 90-day release measurement points. The observed burst and steady release measurements were as expected.
Embodiment 3
[0338] Embodiment 3: the in vivo tolerance test of lyophilized composition
[0339] This study was designed to evaluate the ocular tolerance of CC-101 in non-human primates following intravitreal (IVT) injection to establish doses for efficacy testing. This was achieved by slit-lamp examination, retinal imaging, tonometry, and clinicopathology after repeated escalation administrations.
[0340] Test Compound Disposal
[0341] During the two-day shipping transit time, the vials were kept on dry ice in a shipping container kept below -20 °C. Vials were then kept at -20°C until thawed at room temperature immediately prior to use. At the time of dosing, a low dose (64 μg / ml) was prepared by adding 1 mL of 0.9% sterile saline to a 5 mL vial of 15CCT1-150709 CC-101 Histidine. The high dose (128 μg / ml) was prepared by adding 0.5 mL of 0.9% sterile saline to a 5 mL vial of 15CCT1-150709 CC-101 histidine.
[0342] Subject recruitment
[0343] Three adult males who performed the ex...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap